Loss of KAP3 decreases intercellular adhesion and impairs intracellular transport of laminin in signet ring cell carcinoma of the stomach
- PMID: 35322078
- PMCID: PMC8943207
- DOI: 10.1038/s41598-022-08904-8
Loss of KAP3 decreases intercellular adhesion and impairs intracellular transport of laminin in signet ring cell carcinoma of the stomach
Abstract
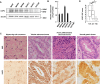
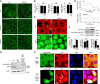
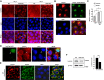
Signet-ring cell carcinoma (SRCC) is a unique subtype of gastric cancer that is impaired for cell-cell adhesion. The pathogenesis of SRCC remains unclear. Here, we show that expression of kinesin-associated protein 3 (KAP3), a cargo adaptor subunit of the kinesin superfamily protein 3 (KIF3), a motor protein, is specifically decreased in SRCC of the stomach. CRISPR/Cas9-mediated gene knockout experiments indicated that loss of KAP3 impairs the formation of circumferential actomyosin cables by inactivating RhoA, leading to the weakening of cell-cell adhesion. Furthermore, in KAP3 knockout cells, post-Golgi transport of laminin, a key component of the basement membrane, was inhibited, resulting in impaired basement membrane formation. Together, these findings uncover a potential role for KAP3 in the pathogenesis of SRCC of the stomach.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Separable roles for RanGTP in nuclear and ciliary trafficking of a kinesin-2 subunit.J Biol Chem. 2021 Jan-Jun;296:100117. doi: 10.1074/jbc.RA119.010936. Epub 2020 Dec 3. J Biol Chem. 2021. PMID: 33234597 Free PMC article.
-
An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: III. Expressions of EMA, CEA, CA19-9, CDX-2, p53, Ki-67 antigen, TTF-1, vimentin, and p63 in normal mucosa and in 42 cases.Int J Clin Exp Pathol. 2013;6(4):630-8. Epub 2013 Mar 15. Int J Clin Exp Pathol. 2013. PMID: 23573309 Free PMC article.
-
Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge.World J Gastroenterol. 2015 Oct 28;21(40):11428-38. doi: 10.3748/wjg.v21.i40.11428. World J Gastroenterol. 2015. PMID: 26523107 Free PMC article. Review.
-
Potential therapeutic targets discovery by transcriptome analysis of an in vitro human gastric signet ring carcinoma model.Gastric Cancer. 2022 Sep;25(5):862-878. doi: 10.1007/s10120-022-01307-8. Epub 2022 Jun 4. Gastric Cancer. 2022. PMID: 35661943
-
Signet ring cell cancer of stomach and gastro-esophageal junction: molecular alterations, stage-stratified treatment approaches, and future challenges.Langenbecks Arch Surg. 2022 Feb;407(1):87-98. doi: 10.1007/s00423-021-02314-6. Epub 2021 Sep 10. Langenbecks Arch Surg. 2022. PMID: 34505199 Free PMC article. Review.
Cited by
-
Emerging functions of Plakophilin 4 in the control of cell contact dynamics.Cell Commun Signal. 2025 Feb 25;23(1):109. doi: 10.1186/s12964-025-02106-1. Cell Commun Signal. 2025. PMID: 40001215 Free PMC article. Review.
-
Comparative Study of ERK1/2, Bcl2, and Sorcin with Clinicopathological Characteristics of Gastric carcinoma: Tubular Adenocarcinoma Vs Signet Ring Cell Carcinoma.Asian Pac J Cancer Prev. 2025 May 1;26(5):1581-1589. doi: 10.31557/APJCP.2025.26.5.1581. Asian Pac J Cancer Prev. 2025. PMID: 40439369 Free PMC article.
-
KAP is the neuronal organelle adaptor for Kinesin-2 KIF3AB and KIF3AC.Mol Biol Cell. 2022 Dec 1;33(14):ar133. doi: 10.1091/mbc.E22-08-0336. Epub 2022 Oct 6. Mol Biol Cell. 2022. PMID: 36200888 Free PMC article.
References
-
- Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965;64:31–49. - PubMed
-
- Parsonnet J, et al. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J. Natl. Cancer. Inst. 1991;83:640–643. - PubMed
-
- Mayer RJ, et al. Gastrointestinal Tract Cancer. In: Longo DL, Fauci AS, Kasper DL, et al., editors. Harrison’s Principles of Internal Medicine. 18. McGraw-Hill Campanies; 2012. pp. 764–776.
-
- Lauwers G, Carneiro F, Graham D, Curado M, Franceschi S. Classification of Tumours of the Digestive System. 4. IARC Press; 2010. pp. 45–58.
-
- Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English. Gastric Cancer. 2011;14:101–112. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

