Glyoxylate protects against cyanide toxicity through metabolic modulation
- PMID: 35322094
- PMCID: PMC8943054
- DOI: 10.1038/s41598-022-08803-y
Glyoxylate protects against cyanide toxicity through metabolic modulation
Abstract
Although cyanide's biological effects are pleiotropic, its most obvious effects are as a metabolic poison. Cyanide potently inhibits cytochrome c oxidase and potentially other metabolic enzymes, thereby unleashing a cascade of metabolic perturbations that are believed to cause lethality. From systematic screens of human metabolites using a zebrafish model of cyanide toxicity, we have identified the TCA-derived small molecule glyoxylate as a potential cyanide countermeasure. Following cyanide exposure, treatment with glyoxylate in both mammalian and non-mammalian animal models confers resistance to cyanide toxicity with greater efficacy and faster kinetics than known cyanide scavengers. Glyoxylate-mediated cyanide resistance is accompanied by rapid pyruvate consumption without an accompanying increase in lactate concentration. Lactate dehydrogenase is required for this effect which distinguishes the mechanism of glyoxylate rescue as distinct from countermeasures based solely on chemical cyanide scavenging. Our metabolic data together support the hypothesis that glyoxylate confers survival at least in part by reversing the cyanide-induced redox imbalances in the cytosol and mitochondria. The data presented herein represent the identification of a potential cyanide countermeasure operating through a novel mechanism of metabolic modulation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
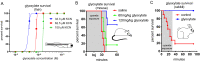
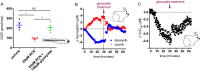

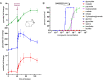

References
-
- Institute SIPR. In The Problem of Chemical and Biological Warfare: The Rise of CB Weapons. (eds. Robinson, J. P.) (Almqvist & Wiksell, 1971).
-
- Winek CL, Fusia E, Collom WD, Shanor SP. Cyanide poisoning as a mode of suicide. Forensic Sci. 1978;11(1):51–55. - PubMed
-
- Cummings TF. The treatment of cyanide poisoning. Occup. Med.-Oxford. 2004;54(2):82–85. - PubMed
-
- Baud FJ, Barriot P, Toffis V, Riou B, Vicaut E, Lecarpentier Y, et al. Elevated blood cyanide concentrations in victims of smoke inhalation. N. Engl. J. Med. 1991;325(25):1761–1766. - PubMed

