A four specimen-pooling scheme reliably detects SARS-CoV-2 and influenza viruses using the BioFire FilmArray Respiratory Panel 2.1
- PMID: 35322125
- PMCID: PMC8942994
- DOI: 10.1038/s41598-022-09039-6
A four specimen-pooling scheme reliably detects SARS-CoV-2 and influenza viruses using the BioFire FilmArray Respiratory Panel 2.1
Abstract
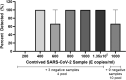
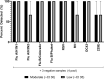
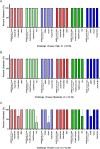
The COVID-19 pandemic required increased testing capacity, enabling rapid case identification and effective contract tracing to reduce transmission of disease. The BioFire FilmArray is a fully automated nucleic acid amplification test system providing specificity and sensitivity associated with gold standard molecular methods. The FilmArray Respiratory Panel 2.1 targets 22 viral and bacterial pathogens, including SARS-CoV-2 and influenza virus. While each panel provides a robust output of information regarding pathogen detection, the specimen throughput is low. This study evaluates the FilmArray Respiratory Panel 2.1 using 33 pools of contrived nasal samples and 22 pools of clinical nasopharyngeal specimens to determine the feasibility of increasing testing capacity, while maintaining detection of both SARS-CoV-2 and influenza virus. We observed 100% detection and 90% positive agreement for SARS-CoV-2 and 98% detection and 95% positive agreement for influenza viruses with pools of contrived or clinical specimens, respectively. While discordant results were mainly attributed to loss in sensitivity, the sensitivity of the pooling assay was well within accepted limits of detection for a nucleic acid amplification test. Overall, this study provides evidence supporting the use of pooling patient specimens, one in four with the FilmArray Respiratory Panel 2.1 for the detection of SARS-CoV-2 and influenza virus.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- BioFire Diagnostics LLC. BioFire Respiratory Panel 2.1 (RP2.1) de novo Instructions for Use. BFR0000-8579-01. https://www.biofiredx.qarad.eifu.online/ITI/CA/en/all?keycode=ITI0105 (2021).
-
- BioFire Diagnostics LLC. Technical Note: BioFire® Respiratory Panels (RP2.1, RP2.1plus and RP2.1-EZ) SARS-CoV-2 Reactivity. BFR0001-2371-06. https://docs.biofiredx.com/wp-content/uploads/BFR0001-2371-06-SARS-CoV-2... (2021).
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

