Accelerated remodeling of the mesophyll-bundle sheath interface in the maize C4 cycle mutant leaves
- PMID: 35322159
- PMCID: PMC8943126
- DOI: 10.1038/s41598-022-09135-7
Accelerated remodeling of the mesophyll-bundle sheath interface in the maize C4 cycle mutant leaves
Abstract
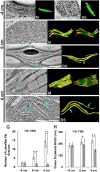
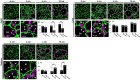
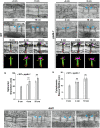
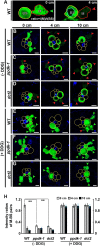
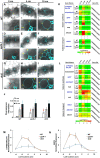
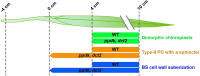
C4 photosynthesis in the maize leaf involves the exchange of organic acids between mesophyll (M) and the bundle sheath (BS) cells. The transport is mediated by plasmodesmata embedded in the suberized cell wall. We examined the maize Kranz anatomy with a focus on the plasmodesmata and cell wall suberization with microscopy methods. In the young leaf zone where M and BS cells had indistinguishable proplastids, plasmodesmata were simple and no suberin was detected. In leaf zones where dimorphic chloroplasts were evident, the plasmodesma acquired sphincter and cytoplasmic sleeves, and suberin was discerned. These modifications were accompanied by a drop in symplastic dye mobility at the M-BS boundary. We compared the kinetics of chloroplast differentiation and the modifications in M-BS connectivity in ppdk and dct2 mutants where C4 cycle is affected. The rate of chloroplast diversification did not alter, but plasmodesma remodeling, symplastic transport inhibition, and cell wall suberization were observed from younger leaf zone in the mutants than in wild type. Our results indicate that inactivation of the C4 genes accelerated the changes in the M-BS interface, and the reduced permeability suggests that symplastic transport between M and BS could be regulated for normal operation of C4 cycle.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
The Metabolite Pathway between Bundle Sheath and Mesophyll: Quantification of Plasmodesmata in Leaves of C3 and C4 Monocots.Plant Cell. 2016 Jun;28(6):1461-71. doi: 10.1105/tpc.16.00155. Epub 2016 Jun 10. Plant Cell. 2016. PMID: 27288224 Free PMC article.
-
Closure of plasmodesmata in maize (Zea mays) at low temperature: a new mechanism for inhibition of photosynthesis.Ann Bot. 2010 Nov;106(5):675-86. doi: 10.1093/aob/mcq169. Epub 2010 Sep 29. Ann Bot. 2010. PMID: 20880933 Free PMC article.
-
Cytological evidence of BSD2 functioning in both chloroplast division and dimorphic chloroplast formation in maize leaves.BMC Plant Biol. 2020 Jan 9;20(1):17. doi: 10.1186/s12870-019-2219-7. BMC Plant Biol. 2020. PMID: 31918680 Free PMC article.
-
C4 rice engineering, beyond installing a C4 cycle.Plant Physiol Biochem. 2024 Jan;206:108256. doi: 10.1016/j.plaphy.2023.108256. Epub 2023 Dec 7. Plant Physiol Biochem. 2024. PMID: 38091938 Review.
-
Insights into the regulation of C4 leaf development from comparative transcriptomic analysis.Curr Opin Plant Biol. 2016 Apr;30:1-10. doi: 10.1016/j.pbi.2015.12.011. Epub 2016 Jan 30. Curr Opin Plant Biol. 2016. PMID: 26828378 Review.
Cited by
-
Identification of maize genes that condition early systemic infection of sugarcane mosaic virus through single-cell transcriptomics.Plant Commun. 2025 May 12;6(5):101297. doi: 10.1016/j.xplc.2025.101297. Epub 2025 Mar 4. Plant Commun. 2025. PMID: 40045576 Free PMC article.
-
Phytic acid is an available phosphorus source for maize plants in juvenile phase belonging to two populations with different breeding backgrounds.BMC Plant Biol. 2025 Apr 3;25(1):425. doi: 10.1186/s12870-025-06431-y. BMC Plant Biol. 2025. PMID: 40181321 Free PMC article.
-
Plasmodesmal connectivity in C4 Gynandropsis gynandra is induced by light and dependent on photosynthesis.New Phytol. 2024 Jan;241(1):298-313. doi: 10.1111/nph.19343. Epub 2023 Oct 26. New Phytol. 2024. PMID: 37882365 Free PMC article.
-
Trafficking Processes and Secretion Pathways Underlying the Formation of Plant Cuticles.Front Plant Sci. 2022 Jan 5;12:786874. doi: 10.3389/fpls.2021.786874. eCollection 2021. Front Plant Sci. 2022. PMID: 35069645 Free PMC article.
References
-
- Taniguchi, M. & Cousins, A. B. The Leaf: A Platform for Performing Photosynthesis (eds. Terashima, I., & Adams Iii, W.W.). 255–279. (Springer, 2018).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

