Generation and characterization of hair-bearing skin organoids from human pluripotent stem cells
- PMID: 35322210
- PMCID: PMC10461778
- DOI: 10.1038/s41596-022-00681-y
Generation and characterization of hair-bearing skin organoids from human pluripotent stem cells
Erratum in
-
Author Correction: Generation and characterization of hair-bearing skin organoids from human pluripotent stem cells.Nat Protoc. 2023 Dec;18(12):3975. doi: 10.1038/s41596-023-00884-x. Nat Protoc. 2023. PMID: 37612404 No abstract available.
Abstract
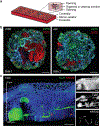
Human skin uses millions of hairs and glands distributed across the body surface to function as an external barrier, thermoregulator and stimuli sensor. The large-scale generation of human skin with these appendages would be beneficial, but is challenging. Here, we describe a detailed protocol for generating hair-bearing skin tissue entirely from a homogeneous population of human pluripotent stem cells in a three-dimensional in vitro culture system. Defined culture conditions are used over a 2-week period to induce differentiation of pluripotent stem cells to surface ectoderm and cranial neural crest cells, which give rise to the epidermis and dermis, respectively, in each organoid unit. After 60 d of incubation, the skin organoids produce hair follicles. By day ~130, the skin organoids reach full complexity and contain stratified skin layers, pigmented hair follicles, sebaceous glands, Merkel cells and sensory neurons, recapitulating the cell composition and architecture of fetal skin tissue at week 18 of gestation. Skin organoids can be maintained in culture using this protocol for up to 150 d, enabling the organoids to be used to investigate basic skin biology, model disease and, further, reconstruct or regenerate skin tissue.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
COMPETING INTERESTS
J.L. and K.R.K, with the Indiana University Research and Technology Corporation, have a patent covering the entire skin organoid induction method (US11021688B2), which is licensed to STEMCELL Technologies, Inc. for research use. The other authors declare no competing interests.
Figures
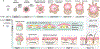




Similar articles
-
Hair-bearing human skin generated entirely from pluripotent stem cells.Nature. 2020 Jun;582(7812):399-404. doi: 10.1038/s41586-020-2352-3. Epub 2020 Jun 3. Nature. 2020. PMID: 32494013 Free PMC article.
-
Hair Follicle Development in Mouse Pluripotent Stem Cell-Derived Skin Organoids.Cell Rep. 2018 Jan 2;22(1):242-254. doi: 10.1016/j.celrep.2017.12.007. Cell Rep. 2018. PMID: 29298425 Free PMC article.
-
An optimized protocol for generating appendage-bearing skin organoids from human-induced pluripotent stem cells.Biol Methods Protoc. 2024 Mar 22;9(1):bpae019. doi: 10.1093/biomethods/bpae019. eCollection 2024. Biol Methods Protoc. 2024. PMID: 38605978 Free PMC article. Review.
-
Skin organoids: A new human model for developmental and translational research.Exp Dermatol. 2021 Apr;30(4):613-620. doi: 10.1111/exd.14292. Epub 2021 Feb 18. Exp Dermatol. 2021. PMID: 33507537 Free PMC article.
-
The adult hair follicle: cradle for pluripotent neural crest stem cells.Birth Defects Res C Embryo Today. 2004 Jun;72(2):162-72. doi: 10.1002/bdrc.20008. Birth Defects Res C Embryo Today. 2004. PMID: 15269890 Review.
Cited by
-
Human-Induced Pluripotent Stem Cells in Plastic and Reconstructive Surgery.Int J Mol Sci. 2024 Feb 3;25(3):1863. doi: 10.3390/ijms25031863. Int J Mol Sci. 2024. PMID: 38339142 Free PMC article. Review.
-
Vascularized 3D Human Skin Models in the Forefront of Dermatological Research.Adv Healthc Mater. 2024 Apr;13(9):e2303351. doi: 10.1002/adhm.202303351. Epub 2024 Feb 1. Adv Healthc Mater. 2024. PMID: 38277705 Free PMC article. Review.
-
Diversity of human skin three-dimensional organotypic cultures.Curr Opin Genet Dev. 2024 Dec;89:102275. doi: 10.1016/j.gde.2024.102275. Epub 2024 Nov 12. Curr Opin Genet Dev. 2024. PMID: 39536613 Review.
-
Advancements in 3D skin bioprinting: processes, bioinks, applications and sensor integration.Int J Extrem Manuf. 2025 Feb 1;7(1):012009. doi: 10.1088/2631-7990/ad878c. Epub 2024 Nov 19. Int J Extrem Manuf. 2025. PMID: 39569402 Free PMC article. Review.
-
Modelling inner ear development and disease using pluripotent stem cells - a pathway to new therapeutic strategies.Dis Model Mech. 2022 Nov 1;15(11):dmm049593. doi: 10.1242/dmm.049593. Epub 2022 Nov 4. Dis Model Mech. 2022. PMID: 36331565 Free PMC article. Review.
References
-
- Dąbrowska AK et al. The relationship between skin function, barrier properties, and body-dependent factors. Ski. Res. Technol. 24, 165–174 (2018). - PubMed
-
- Kolarsick PAJ, Kolarsick MA & Goodwin C Anatomy and physiology of the skin. J. Dermatol. Nurs. Assoc. 3, 203–213 (2011).
-
- Nose H, Kamijo Y & Masuki S Chapter 25—interactions between body fluid homeostasis and thermoregulation in humans. Handb. Clin. Neurol. 156, 417–429 (2018). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

