Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer
- PMID: 35322232
- PMCID: PMC8967713
- DOI: 10.1038/s41586-022-04508-4
Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer
Abstract
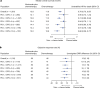
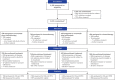
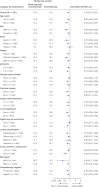
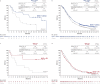
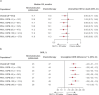
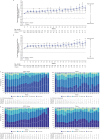
Standard first-line chemotherapy results in disease progression and death within one year in most patients with human epidermal growth factor receptor 2 (HER2)-negative gastro-oesophageal adenocarcinoma1-4. Nivolumab plus chemotherapy demonstrated superior overall survival versus chemotherapy at 12-month follow-up in gastric, gastro-oesophageal junction or oesophageal adenocarcinoma in the randomized, global CheckMate 649 phase 3 trial5 (programmed death ligand-1 (PD-L1) combined positive score ≥5 and all randomized patients). On the basis of these results, nivolumab plus chemotherapy is now approved as a first-line treatment for these patients in many countries6. Nivolumab and the cytotoxic T-lymphocyte antigen-4 (CTLA-4) inhibitor ipilimumab have distinct but complementary mechanisms of action that contribute to the restoration of anti-tumour T-cell function and induction of de novo anti-tumour T-cell responses, respectively7-11. Treatment combining 1 mg kg-1 nivolumab with 3 mg kg-1 ipilimumab demonstrated clinically meaningful anti-tumour activity with a manageable safety profile in heavily pre-treated patients with advanced gastro-oesophageal cancer12. Here we report both long-term follow-up results comparing nivolumab plus chemotherapy versus chemotherapy alone and the first results comparing nivolumab plus ipilimumab versus chemotherapy alone from CheckMate 649. After the 24.0-month minimum follow-up, nivolumab plus chemotherapy continued to demonstrate improvement in overall survival versus chemotherapy alone in patients with PD-L1 combined positive score ≥5 (hazard ratio 0.70; 95% confidence interval 0.61, 0.81) and all randomized patients (hazard ratio 0.79; 95% confidence interval 0.71, 0.88). Overall survival in patients with PD-L1 combined positive score ≥ 5 for nivolumab plus ipilimumab versus chemotherapy alone did not meet the prespecified boundary for significance. No new safety signals were identified. Our results support the continued use of nivolumab plus chemotherapy as standard first-line treatment for advanced gastro-oesophageal adenocarcinoma.
© 2022. The Author(s).
Conflict of interest statement
K.S. reports receiving personal fees for advisory roles from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and Takeda; receiving advisory role or research funding from Astellas Pharma, Eli Lilly, Ono Pharmaceutical, Merck Pharmaceutical, and Taiho Pharmaceutical; receiving honoraria (lecture fees) from AbbVie, Novartis, and Yakult Honsha; and receiving research funding from Amgen, Chugai Pharma, Daiichi Sankyo, Dainippon Sumitomo Pharma, Medi Science, and Eisai, outside the submitted work. J.A.A. reports receiving research grants from Amgen, Astellas Pharma, Bristol Myers Squibb, Daiichi Sankyo, Delta-Fly Pharma, Gilead Sciences, Lilly/ImClone, Merck, Novartis, ProLynx, Roche/Genentech, Taiho Pharmaceutical, Takeda, and Zymeworks; serving as a consultant or in an advisory role for American Cancer Society, BeiGene, Bristol Myers Squibb, Insys Therapeutics, Merck, and Vaccinogen; receiving royalties from or holding patents and other intellectual property with Amgen, Bristol Myers Squibb, Genentech, Lilly, Medimmune, Merck, Roche, and Taiho Pharmaceutical; and receiving honoraria from Acrotech BioPharma, Aduro Biotech, Amgen, Astellas Pharma, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, DAVA Pharmaceuticals, Fresenius Kabi, Gilead Sciences, Grail, Lilly, Merck, Novartis, Servier, and Zymeworks, outside the submitted work. M.M. reports receiving research grants from Amgen, Leap Therapeutics, Merck Serono, and Merck Sharp & Dohme; serving as a consultant or in an advisory role for Amgen, Bayer, Beigene, Bristol Myers Squibb, Lilly, Merck Serono, Merck Sharp & Dohme, Pfizer, Roche, Servier, and Taiho Pharmaceutical; receiving travel and accommodation expenses from American Society of Clinical Oncology, Amgen, Bayer, European Society for Medical Oncology, German Cancer Society, Merck Serono, Merck Sharp & Dohme, and Roche; and receiving honoraria from Amgen, AstraZeneca/MedImmune, Bristol Myers Squibb, Merck Serono, Merck Sharp & Dohme Oncology, Roche/Genentech, Pierre Fabre, Sanofi, and Servier, outside the submitted work. M.G. reports receiving research grants from Bristol Myers Squibb and Novartis; receiving speakers’ bureau fees from Bayer, Bristol Myers Squibb, and Merck; receiving travel and accomodations expenses from Roche; and serving as a consultant or in an advisory role for Merck Sharp & Dohme and Roche, outside the submitted work. C.G. reports receiving research grants from Bristol Myers Squibb and Merck Sharp & Dohme; receiving speakers’ bureau fees from AstraZeneca, Merck Sharp & Dohme, and Novartis; receiving travel and accomodation expenses from Roche; serving as a consultant or in an advisory role for Merck Sharp & Dohme, Novartis, and Roche; and providing expert testimony for AstraZeneca, outside the submitted work. L.S. reports receiving research grants from Beijing Xiantong Biomedical Technology, Qilu Pharmaceutical, ZaiLab Pharmaceutical (Shanghai), Beihai Kangcheng (Beijing) Medical Technology, Jacobio Pharmaceuticals, and Beijing Xiantong Biomedical Technology; receiving consulting fees from Boehringer Ingelheim, Haichuang Pharmaceutical, Herbour Biomed, Merck, Merck Sharp & Dohme, and Mingji Biopharmaceutical; receiving speakers’ fees from CSTONE Pharmaceutical, Hutchison Whampoa, Hengrui, and ZaiLab; and participating on a Data Safety Monitoring Board or Advisory Board for Bristol Myers Squibb, CSTONE Pharmaceutical, Rongchang Pharmaceutical, and ZaiLab, outside the submitted work. K.Y. reports receiving research grants from Boehringer Ingelheim, Bristol Myers Squibb, Chugai Pharma, Daiichi Sankyo, Eisai, Gilead Sciences, Lilly, Merck Sharp & Dohme Oncology, Ono Pharmaceutical, Sanofi, Taiho Pharmaceutical, and Yakult Honsha; receiving speakers’ bureau fees from Bristol Myers Squibb Japan, Chugai Pharma, Daiichi Sankyo, Lilly, Merck, Ono Pharmaceutical, Taiho Pharmaceutical, and Takeda; and serving as a consultant or in an advisory role for Bristol Myers Squibb Japan and Daiichi Sankyo, outside the submitted work. L.W. reports receiving research grants from National Cancer Research Institute; receiving speakers’ bureau fees from Amgen, Roche, Sanofi, and Servier; and serving in a consulting or advisory role for Amgen and Servier, outside the submitted work. T.S. has no competing interests to disclose. A.B. has no competing interests to disclose. T.L. has no competing interests to disclose. M.T. reports receiving research funding from Celgene; receiving honoraria from Bristol Myers Squibb, Celgene, Eisai, Merck, Pfizer, and Taiho Pharmaceutical; and serving in a consulting or advisory role for Bayer, Bristol Myers Squibb, Celgene, Eisai, Merck, Taiho Pharmaceutical, and Takeda, outside the submitted work. E.E. reports receiving research funding from Bristol Myers Squibb and Zymeworks; serving as a consultant or in an advisory role for Bristol Myers Squibb, Zymeworks, and Adaptimmune; and having an immediate family member employed by Merck, outside the submitted work. R.B. reports serving as a medical adviser for AstraZeneca, Bristol Myers Squibb, Merck Serono, Novartis, and Pfizer; receiving clinical research funding from Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, and Roche; and serving as a speaker for AstraZeneca, Bristol Myers Squibb, Merck, and Pfizer, during the conduct of the study. T.Z. reports serving as a consultant or in an advisory role for Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, and Roche, outside the submitted work. S.d.A. reports receiving honoraria from AstraZeneca, Bristol Myers Squibb, Genentech, Merck Sharp & Dohme, Novartis, and Roche, outside the sumbitted work. R.K. reports receiving research grants from Amgen, Astellas, AstraZeneca, Athenex, Bristol Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Nektar, Novartis, Pfizer, Roche, and Sanofi; receiving non-financial support from Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer, and Roche; and receiving personal fees from Astellas, Bristol Myers Squibb, Gador, Merck Sharp & Dohme, Novartis, and Pfizer, outside the submitted work. R.P.-C. has no competing interests to disclose. M.S. reports receiving research funding from Abbvie, Astellas Pharma, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Pfizer/EMD Serono, Regeneron, and Roche; and receiving travel, accommodations, and expenses from Bristol Myers Squibb, outside the submitted work. J.M.C. reports receiving research funding to his institution from Abbvie, Merus, Roche, and Bristol Myers Squibb; receiving research funding from Merck, AstraZeneca, Esperas Pharma, Bayer, and Tesaro; receiving consulting fees from Bristol Myers Squibb; receiving an honorarium for advisory board participation from Syros Pharmaceuticals; and receiving travel funding from Bristol Myers Squibb, outside the submitted work. P.Y. has no competing interests to disclose. K.F. has no competing interests to disclose. M.K. reports serving in an advisory role for Bristol Myers Squibb, Merck Sharp & Dohme, Ipsen, Roche, Sandoz, Sanofi, and Servier, outside the submitted work. V.P., M. Lei, H.X., K.K. and M. Li report being employees of Bristol Myers Squibb. Y.Y.J. reports receiving research funding from Bayer, Bristol Myers Squibb, Cycle for Survival, Department of Defense, Fred’s Team, Genentech/Roche Lilly, Merck & Co, National Cancer Institute, and Rgenix; serving as a consultant or in an advisory role for Basilea Pharmaceutical, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Imugene, Lilly, Merck, Merck Serono, Michael J Hennessy Associates, Paradigm Medical Communications, Pfizer, Rgenix, Seagen, and Zymeworks; receiving stock options from Rgenix; and nonfinancial relationships with Clinical Care Options, Axis Medical Education, and Research to Practice, outside the submitted work.
Figures






References
-
- Catenacci DVT, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1467–1482. doi: 10.1016/S1470-2045(17)30566-1. - DOI - PMC - PubMed
-
- Fuchs CS, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:420–435. doi: 10.1016/S1470-2045(18)30791-5. - DOI - PubMed
-
- Janjigian YY, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

