Pharmacological Rescue with SR8278, a Circadian Nuclear Receptor REV-ERBα Antagonist as a Therapy for Mood Disorders in Parkinson's Disease
- PMID: 35322351
- PMCID: PMC9226214
- DOI: 10.1007/s13311-022-01215-w
Pharmacological Rescue with SR8278, a Circadian Nuclear Receptor REV-ERBα Antagonist as a Therapy for Mood Disorders in Parkinson's Disease
Erratum in
-
Correction to: Pharmacological Rescue With SR8278, A Circadian Nuclear Receptor REV-ERBα Antagonist As A Therapy for Mood Disorders In Parkinson's Disease.Neurotherapeutics. 2022 Jul;19(4):1432. doi: 10.1007/s13311-022-01234-7. Neurotherapeutics. 2022. PMID: 35499682 Free PMC article. No abstract available.
Abstract
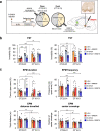
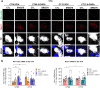
Parkinson's disease is a neurodegenerative disease characterized by progressive dopaminergic neuronal loss. Motor deficits experienced by patients with Parkinson's disease are well documented, but non-motor symptoms, including mood disorders associated with circadian disturbances, are also frequent features. One common phenomenon is "sundowning syndrome," which is characterized by the occurrence of neuropsychiatric symptoms at a specific time (dusk), causing severe quality of life challenges. This study aimed to elucidate the underlying mechanisms of sundowning syndrome in Parkinson's disease and their molecular links with the circadian clock. We demonstrated that 6-hydroxydopamine (6-OHDA)-lesioned mice, as Parkinson's disease mouse model, exhibit increased depression- and anxiety-like behaviors only at dawn (the equivalent of dusk in human). Administration of REV-ERBα antagonist, SR8278, exerted antidepressant and anxiolytic effects in a circadian time-dependent manner in 6-OHDA-lesioned mice and restored the circadian rhythm of mood-related behaviors. 6-OHDA-lesion altered DAergic-specific Rev-erbα and Nurr1 transcription, and atypical binding activities of REV-ERBα and NURR1, which are upstream nuclear receptors for the discrete tyrosine hydroxylase promoter region. SR8278 treatment restored the binding activities of REV-ERBα and NURR1 to the tyrosine hydroxylase promoter and the induction of enrichment of the R/N motif, recognized by REV-ERBα and NURR1, as revealed by ATAC-sequencing; therefore, tyrosine hydroxylase expression was elevated in the ventral tegmental area of 6-OHDA-injected mice, especially at dawn. These results indicate that REV-ERBα is a potential therapeutic target, and its antagonist, SR8278, is a potential drug for mood disorders related to circadian disturbances, namely sundowning syndrome, in Parkinson's disease.
Keywords: Circadian mood regulation; Dopaminergic neuronal loss; Nurr1; Parkinson’s disease; Rev-erbα; Sundowning syndrome.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Abrogation of the Circadian Nuclear Receptor REV-ERBα Exacerbates 6-Hydroxydopamine-Induced Dopaminergic Neurodegeneration.Mol Cells. 2018 Aug 31;41(8):742-752. doi: 10.14348/molcells.2018.0201. Epub 2018 Jul 30. Mol Cells. 2018. PMID: 30078232 Free PMC article.
-
Integration of the nuclear receptor REV-ERBα linked with circadian oscillators in the expressions of Alas1, Ppargc1a, and Il6 genes in rat granulosa cells.Chronobiol Int. 2015;32(6):739-49. doi: 10.3109/07420528.2015.1042582. Epub 2015 Jun 23. Chronobiol Int. 2015. PMID: 26102301
-
Rev-erbα regulate neurogenesis through suppression of Sox2 in neuronal cells to regenerate dopaminergic neurons and abates MPP+ induced neuroinflammation.Free Radic Biol Med. 2024 Oct;223:144-159. doi: 10.1016/j.freeradbiomed.2024.07.025. Epub 2024 Jul 29. Free Radic Biol Med. 2024. PMID: 39084577
-
Implications of Circadian Rhythm in Dopamine and Mood Regulation.Mol Cells. 2017 Jul 31;40(7):450-456. doi: 10.14348/molcells.2017.0065. Epub 2017 Jul 31. Mol Cells. 2017. PMID: 28780783 Free PMC article. Review.
-
The nuclear receptor REV-ERBα integrates circadian clock and energy metabolism.Yi Chuan. 2023 Feb 20;45(2):99-114. doi: 10.16288/j.yczz.22-310. Yi Chuan. 2023. PMID: 36927658 Review.
Cited by
-
Challenges and Opportunities in Exploring Non-Motor Symptoms in 6-Hydroxydopamine Models of Parkinson's Disease: A Systematic Review.J Neurochem. 2025 Feb;169(2):e70008. doi: 10.1111/jnc.70008. J Neurochem. 2025. PMID: 39901598 Free PMC article.
-
Circadian rhythm disruption: a potential trigger in Parkinson's disease pathogenesis.Front Cell Neurosci. 2024 Oct 30;18:1464595. doi: 10.3389/fncel.2024.1464595. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39539340 Free PMC article. Review.
-
Targeting NR1D1 in organ injury: challenges and prospects.Mil Med Res. 2023 Dec 11;10(1):62. doi: 10.1186/s40779-023-00495-3. Mil Med Res. 2023. PMID: 38072952 Free PMC article. Review.
-
Circadian Regulation of the Neuroimmune Environment Across the Lifespan: From Brain Development to Aging.J Biol Rhythms. 2023 Oct;38(5):419-446. doi: 10.1177/07487304231178950. Epub 2023 Jun 26. J Biol Rhythms. 2023. PMID: 37357738 Free PMC article. Review.
-
Rev‑erbα: The circadian guardian against NLRP3‑driven liver fibrosis.Mol Med Rep. 2025 Oct;32(4):270. doi: 10.3892/mmr.2025.13635. Epub 2025 Jul 25. Mol Med Rep. 2025. PMID: 40709400 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

