Multiple cycles of granulocyte colony-stimulating factor in decompensated cirrhosis: a double-blind RCT
- PMID: 35322373
- PMCID: PMC8942063
- DOI: 10.1007/s12072-022-10314-x
Multiple cycles of granulocyte colony-stimulating factor in decompensated cirrhosis: a double-blind RCT
Abstract
Background: Liver transplant, the definitive treatment of decompensated cirrhosis (DC), is constrained by donor shortage and long-term complications. Granulocyte colony-stimulating factor (G-CSF) has been explored as an alternative option in open-label studies. This double-blind, randomized, placebo-controlled trial was designed to elucidate the efficacy of G-CSF in DC.
Methods: Seventy patients were randomized to either G-CSF plus standard medical therapy (group A, n = 35) or placebo plus standard medical therapy (group B, n = 35). Primary outcome was 12-month overall survival in patients who received at least one cycle of intervention. Secondary outcomes were mobilization of CD34+ cells at day 6, improvement in Child-Turcotte-Pugh (CTP), and model for end-stage liver disease (MELD), liver stiffness measurement, quality of life, nutrition, hepatic decompensation, infection, hospitalization, and acute kidney injury.
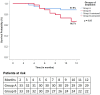
Results: Survival in group A was higher than that in Group B although the difference was not statistically significant (87.9% vs 66.7%; p = 0.053). CD34+ cells at day 6 were significantly higher in group A as compared to baseline (p < 0.001). Ascites control (p = 0.03) and CTP score improvement (p = 0.02) were better in group A at 12-months. Encephalopathy episodes (p = 0.005), infections (p = 0.005) were fewer in group A than group B at 12 months. Other secondary outcomes did not improve post-therapy. There were no treatment-related discontinuations or severe adverse events.
Conclusions: G-CSF therapy is safe. The improvement in survival at 12 months is not statistically significant. Better control of ascites, improvement of CTP score, fewer encephalopathy episodes and decreased rate of infections were observed with G-CSF therapy (NCT03911037). Trials Registration NCT03911037.
Keywords: Ascites; Chronic liver disease; Cirrhosis; End-stage liver disease; G-CSF; Growth factors; Hematopoietic stem cells; Hepatic encephalopathy; Liver regeneration; Portal hypertension; Variceal bleed.
© 2022. Asian Pacific Association for the Study of the Liver.
Conflict of interest statement
Aswath Venkitaraman, Arka De, Nipun Verma, Sunita Kumari, Bidyalaxmi Leishangthem, Ratti Ram Sharma, Naveen Kalra, Sandeep Grover, Virendra Singh declare no conflicting interests and have no financial disclosures.
Figures
Similar articles
-
Role of granulocyte colony stimulating factor in the treatment of cirrhosis of liver: a systematic review.J Int Med Res. 2023 Nov;51(11):3000605231207064. doi: 10.1177/03000605231207064. J Int Med Res. 2023. PMID: 37946367 Free PMC article.
-
Multiple Cycles of Granulocyte Colony-Stimulating Factor Increase Survival Times of Patients With Decompensated Cirrhosis in a Randomized Trial.Clin Gastroenterol Hepatol. 2021 Feb;19(2):375-383.e5. doi: 10.1016/j.cgh.2020.02.022. Epub 2020 Feb 21. Clin Gastroenterol Hepatol. 2021. PMID: 32088302 Clinical Trial.
-
Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial.Lancet Gastroenterol Hepatol. 2018 Jan;3(1):25-36. doi: 10.1016/S2468-1253(17)30326-6. Epub 2017 Nov 7. Lancet Gastroenterol Hepatol. 2018. PMID: 29127060 Free PMC article. Clinical Trial.
-
Early cirrhosis and a preserved bone marrow niche favour regenerative response to growth factors in decompensated cirrhosis.Liver Int. 2019 Jan;39(1):115-126. doi: 10.1111/liv.13923. Epub 2018 Aug 10. Liver Int. 2019. PMID: 29962032 Clinical Trial.
-
Granulocyte-colony stimulating factor in acute-on-chronic liver failure: Systematic review and meta-analysis of randomized controlled trials.Gastroenterol Hepatol. 2023 May;46(5):350-359. doi: 10.1016/j.gastrohep.2022.09.007. Epub 2022 Sep 27. Gastroenterol Hepatol. 2023. PMID: 36174797 English, Spanish.
Cited by
-
Recent advances in the prevention and treatment of decompensated cirrhosis and acute-on-chronic liver failure (ACLF) and the role of biomarkers.Gut. 2024 May 10;73(6):1015-1024. doi: 10.1136/gutjnl-2023-330584. Gut. 2024. PMID: 38527788 Free PMC article. Review.
-
Circulating neutrophil anti-pathogen dysfunction in cirrhosis.JHEP Rep. 2023 Aug 1;5(11):100871. doi: 10.1016/j.jhepr.2023.100871. eCollection 2023 Nov. JHEP Rep. 2023. PMID: 37822786 Free PMC article. Review.
-
Granulocyte colony-stimulating factor with or without stem or progenitor cell or growth factors infusion for people with compensated or decompensated advanced chronic liver disease.Cochrane Database Syst Rev. 2023 Jun 6;6(6):CD013532. doi: 10.1002/14651858.CD013532.pub2. Cochrane Database Syst Rev. 2023. PMID: 37278488 Free PMC article.
-
Expert consensus on the diagnosis and treatment of end-stage liver disease complicated by infections.Hepatol Int. 2024 Jun;18(3):817-832. doi: 10.1007/s12072-023-10637-3. Epub 2024 Mar 9. Hepatol Int. 2024. PMID: 38460060 Review.
-
Role of granulocyte colony stimulating factor in the treatment of cirrhosis of liver: a systematic review.J Int Med Res. 2023 Nov;51(11):3000605231207064. doi: 10.1177/03000605231207064. J Int Med Res. 2023. PMID: 37946367 Free PMC article.
References
-
- De A, Kumari S, Singh A, Kaur A, Sharma R, Bhalla A, et al. Multiple cycles of granulocyte colony-stimulating factor increase survival times of patients with decompensated cirrhosis in a randomized trial. Clin Gastroenterol Hepatol. 2021;19(2):375–383.e5. doi: 10.1016/j.cgh.2020.02.022. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical