VIP plasma levels associate with survival in severe COVID-19 patients, correlating with protective effects in SARS-CoV-2-infected cells
- PMID: 35322471
- PMCID: PMC9088587
- DOI: 10.1002/JLB.5COVA1121-626R
VIP plasma levels associate with survival in severe COVID-19 patients, correlating with protective effects in SARS-CoV-2-infected cells
Abstract
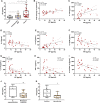
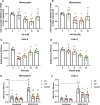
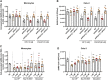
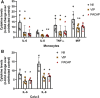
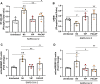
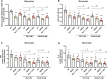
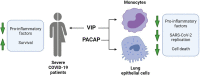
Infection by SARS-CoV-2 may elicit uncontrolled and damaging inflammatory responses. Thus, it is critical to identify compounds able to inhibit virus replication and thwart the inflammatory reaction. Here, we show that the plasma levels of the immunoregulatory neuropeptide VIP are elevated in patients with severe COVID-19, correlating with reduced inflammatory mediators and with survival on those patients. In vitro, vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP), highly similar neuropeptides, decreased the SARS-CoV-2 RNA content in human monocytes and viral production in lung epithelial cells, also reducing cell death. Both neuropeptides inhibited the production of proinflammatory mediators in lung epithelial cells and in monocytes. VIP and PACAP prevented in monocytes the SARS-CoV-2-induced activation of NF-kB and SREBP1 and SREBP2, transcriptions factors involved in proinflammatory reactions and lipid metabolism, respectively. They also promoted CREB activation, a transcription factor with antiapoptotic activity and negative regulator of NF-kB. Specific inhibition of NF-kB and SREBP1/2 reproduced the anti-inflammatory, antiviral, and cell death protection effects of VIP and PACAP. Our results support further clinical investigations of these neuropeptides against COVID-19.
Keywords: COVID-19; PACAP; SARS-CoV-2; VIP; neuropeptides.
©2022 Society for Leukocyte Biology.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

