Torsade de pointes caused by citalopram during the pacemaker battery-depletion phase: A case report
- PMID: 35322503
- PMCID: PMC9296796
- DOI: 10.1111/anec.12936
Torsade de pointes caused by citalopram during the pacemaker battery-depletion phase: A case report
Abstract
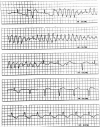
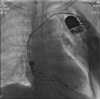
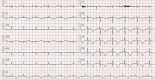
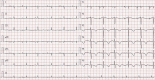
Drug-induced QT prolongation, primarily antiarrhythmic drugs, is a common cause of torsade de pointes (TdP). Although there have been previous reports of drug-induced TdP in patients, it has not been well documented when caused by citalopram during the pacemaker battery-depletion phase. To improve delirium recognition, we report a case of citalopram-induced TdP during the pacemaker battery-depletion phase. An 84-year-old Chinese female was brought to the hospital presenting recurrent syncope. She lost consciousness and was admitted after her syncope TdP was documented. Her pacemaker was inspected and found to be operating in an extremely ineffective manner. Although she had prolonged QT interval after the pacemaker was replaced, she did not suffer another syncope attack, and ECG monitoring revealed no cardiac arrhythmia or TdP. During her admission, she was treated with citalopram for depression. Citalopram was discontinued when the QT interval shortened progressively. In this study, we described a case of citalopram-induced TdP during the depletion phase of a pacemaker battery. This case should serve as a cautionary lesson to clinicians to avoid using citalopram during the pacemaker battery-depletion phase.
Keywords: case report; citalopram; pacemaker battery depletion; torsade de pointes.
© 2022 The Authors. Annals of Noninvasive Electrocardiology published by Wiley Periodicals LLC.
Conflict of interest statement
There are no conflicts of interest reported by any of the authors in relation to the research, authorship, and/or publication of this article.
Figures
References
-
- Crépeau‐Gendron, G. , Brown, H. , Shorey, C. , Madan, R. , Szabuniewicz, C. , Koh, S. , Veinish, S. , & Mah, L. (2019). Association between citalopram, escitalopram and QTc prolongation in a real‐world geriatric setting. Journal of Affective Disorders, 250, 341–345. 10.1016/j.jad.2019.02.060 - DOI - PubMed
-
- Drew, B. , Ackerman, M. , Funk, M. , Gibler, W. , Kligfield, P. , Menon, V. , Philippides, G. J. , Roden, D. M. , & Zareba, W. (2010). Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation, 121(8), 1047–1060. 10.1161/circulationaha.109.192704 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

