Kidney Decellularized Extracellular Matrix Enhanced the Vascularization and Maturation of Human Kidney Organoids
- PMID: 35322595
- PMCID: PMC9130892
- DOI: 10.1002/advs.202103526
Kidney Decellularized Extracellular Matrix Enhanced the Vascularization and Maturation of Human Kidney Organoids
Abstract
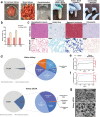
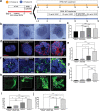
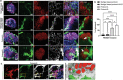
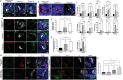
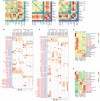
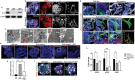
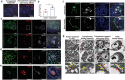
Kidney organoids derived from human pluripotent stem cells (hPSCs) have extensive potential for disease modelling and regenerative medicine. However, the limited vascularization and immaturity of kidney organoids have been still remained to overcome. Extracellular matrix (ECM) can provide mechanical support and a biochemical microenvironment for cell growth and differentiation. Here in vitro methods using a kidney decellularized extracellular matrix (dECM) hydrogel to culture hPSC-derived kidney organoids, which have extensive vascular network and their own endothelial cells, are reported. Single-cell transcriptomics reveal that the vascularized kidney organoids cultured using the kidney dECM have more mature patterns of glomerular development and higher similarity to human kidney than those cultured without the kidney dECM. Differentiation of α-galactosidase A (GLA)-knock-out hPSCs generated using CRISPR/Cas9 into kidney organoids by the culture method using kidney dECM efficiently recapitulate Fabry nephropathy with vasculopathy. Transplantation of kidney organoids with kidney dECM into kidney of mouse accelerates the recruitment of endothelial cells from the host mouse kidney and maintains vascular integrity with the more organized slit diaphragm-like structures than those without kidney dECM. The kidney dECM methodology for inducing extensive vascularization and maturation of kidney organoids can be applied to studies for kidney development, disease modeling, and regenerative medicine.
Keywords: extracellular matrix; kidney; organoid; vascularization.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Taguchi A., Kaku Y., Ohmori T., Sharmin S., Ogawa M., Sasaki H., Nishinakamura R., Cell Stem Cell 2014, 14, 53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
