PSG7 and 9 (Pregnancy-Specific β-1 Glycoproteins 7 and 9): Novel Biomarkers for Preeclampsia
- PMID: 35322669
- PMCID: PMC9075453
- DOI: 10.1161/JAHA.121.024536
PSG7 and 9 (Pregnancy-Specific β-1 Glycoproteins 7 and 9): Novel Biomarkers for Preeclampsia
Abstract
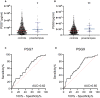
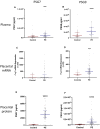
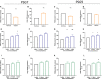
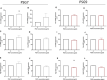
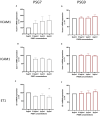
Background Preeclampsia is pregnancy specific, involving significant maternal endothelial dysfunction. Predictive biomarkers are lacking. We evaluated the biomarker potential, expression, and function of PSG7 (pregnancy-specific β-1 glycoprotein 7) and PSG9 (pregnancy-specific β-1 glycoprotein 9) in preeclampsia. Methods and Results At 36 weeks gestation preceding term preeclampsia diagnosis, PSG7 and PSG9 (in Australian cohorts of n=918 and n=979, respectively) were significantly increased before the onset of term preeclampsia (PSG7, P=0.013; PSG9, P=0.0011). In samples collected at 28 to 32 weeks from those with preexisting cardiovascular disease and at high risk of preeclampsia (Manchester Antenatal Vascular Service, UK cohort, n=235), both PSG7 and PSG9 were also significantly increased preceding preeclampsia onset (PSG7, P<0.0001; PSG9, P=0.0003) relative to controls. These changes were validated in the plasma and placentas of patients with established preeclampsia who delivered at <34 weeks gestation (PSG7, P=0.0008; PSG9, P<0.0001). To examine whether PSG7 and PSG9 are associated with increasing disease severity, we measured them in a cohort from South Africa stratified for this outcome, the PROVE (Preeclampsia Obstetric Adverse Events) cohort (n=72). PSG7 (P=0.0027) and PSG9 (P=0.0028) were elevated among patients who were preeclamptic with severe features (PROVE cohort), but not significantly changed in those without severe features or with eclampsia. In syncytialized first trimester cytotrophoblast stem cells, exposure to TNFα (tumor necrosis factor α) or IL-6 (interleukin 6) significantly increased the expression and secretion of PSG7 and PSG9. In contrast, when we treated primary endothelial cells with recombinant PSG7 and PSG9, we only observed modest changes in Flt-1 (FMS-like tyrosine kinase-1) expression and Plgf (placental growth factor) expression, and no other effects on proangiogenic/antiangiogenic or endothelial dysfunction markers were observed. Conclusions Circulating PSG7 and PSG9 are increased before preeclampsia onset and among those with established disease with their production and release potentially driven by placental inflammation.
Keywords: biomarkers; placenta; preeclampsia; pregnancy; pregnancy specific beta‐1 glycoproteins.
Figures
References
-
- Brümmendorf T, Rathjen FG. Cell adhesion molecules. 1: immunoglobulin superfamily. Protein Profile. 1994;1:951–1058. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

