ChGn-2 Plays a Cardioprotective Role in Heart Failure Caused by Acute Pressure Overload
- PMID: 35322673
- PMCID: PMC9075488
- DOI: 10.1161/JAHA.121.023401
ChGn-2 Plays a Cardioprotective Role in Heart Failure Caused by Acute Pressure Overload
Abstract
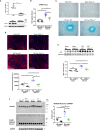
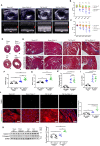
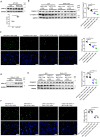
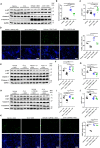
Background Cardiac extracellular matrix is critically involved in cardiac homeostasis, and accumulation of chondroitin sulfate glycosaminoglycans (CS-GAGs) was previously shown to exacerbate heart failure by augmenting inflammation and fibrosis at the chronic phase. However, the mechanism by which CS-GAGs affect cardiac functions remains unclear, especially at the acute phase. Methods and Results We explored a role of CS-GAG in heart failure using mice with target deletion of ChGn-2 (chondroitin sulfate N-acetylgalactosaminyltransferase-2) that elongates CS chains of glycosaminoglycans. Heart failure was induced by transverse aortic constriction in mice. The role of CS-GAG derived from cardiac fibroblasts in cardiomyocyte death was analyzed. Cardiac fibroblasts were subjected to cyclic mechanical stretch that mimics increased workload in the heart. Significant CS-GAGs accumulation was detected in the heart of wild-type mice after transverse aortic constriction, which was substantially reduced in ChGn-2-/- mice. Loss of ChGn-2 deteriorated the cardiac dysfunction caused by pressure overload, accompanied by augmented cardiac hypertrophy and increased cardiomyocyte apoptosis. Cyclic mechanical stretch increased ChGn-2 expression and enhanced glycosaminoglycan production in cardiac fibroblasts. Conditioned medium derived from the stretched cardiac fibroblasts showed cardioprotective effects, which was abolished by CS-GAGs degradation. We found that CS-GAGs elicits cardioprotective effects via dual pathway; direct pathway through interaction with CD44, and indirect pathway through binding to and activating insulin-like growth factor-1. Conclusions Our data revealed the cardioprotective effects of CS-GAGs; therefore, CS-GAGs may play biphasic role in the development of heart failure; cardioprotective role at acute phase despite its possible unfavorable role in the advanced phase.
Keywords: cardiac fibroblasts; cardiomyocytes; chondroitin sulfate glycosaminoglycan; extracellular matrix; heart failure; mechanical stretch.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

