Long non-coding RNA MEG3 promotes tumor necrosis factor-alpha induced oxidative stress and apoptosis in interstitial cells of cajal via targeting the microRNA-21 /I-kappa-B-kinase beta axis
- PMID: 35322738
- PMCID: PMC9161977
- DOI: 10.1080/21655979.2022.2054501
Long non-coding RNA MEG3 promotes tumor necrosis factor-alpha induced oxidative stress and apoptosis in interstitial cells of cajal via targeting the microRNA-21 /I-kappa-B-kinase beta axis
Abstract
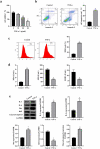
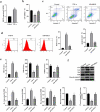
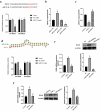
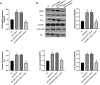
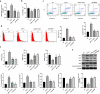
Interstitial Cells of Cajal (ICC) plays a critical role in the peristaltic contractions of the gastrointestinal and urinary tract. The dysfunction and loss of ICC contributes to hypokinetic disease, such as gallstoneand ureteropelvic junction obstruction . In the present study, we identified the underlying driving molecular signals of oxidative stress and apoptosis in ICC. ICC was isolated from small intestine of Balb/c mice, and stimulated with tumor necrosis factor-alpha (TNF-α). MTT and flow cytometry were performed to assess cell viability, apoptosis, and the level of reactive oxygen species in ICC, respectively. The level of malondialdehyde, superoxide dismutase, and glutathione peroxidase in cells were measured to assess oxidative stress. The expression of inflammatory factors (interleukin, IL-1 and IL-6) and apoptosis-related proteins were detected by western blot. We observed that TNF-αinduced inflammation, oxidative stress and cell apoptosis in ICC. By using quantitative real-time PCR , we verified that the expression of long non-coding RNAMEG3 was elevated by TNF-α in ICC. Silencing MEG3 reversed inflammation, oxidative stress, and cell apoptosisin TNF-α-treated ICC. Subsequently, we confirmed that MEG3 sponged cytoprotective miR-21 to upregulate the expression of I-kappa-B-kinase beta (IKKB) and activate the nuclear factor kappa-B (NF-κB) pathway. Both miR-21 overexpression and IKKB knockdown reduced TNF-α-induced above symptoms in ICC. Taken together, we can conclude that MEG3 mediates inflammation, oxidative stress and apoptosis in TNF-α-treated ICC via the miR-21/IKKB-NF-κB axis. The study improves our understanding of the molecular mechanism of ICC reduction related diseases.
Keywords: Interstitial Cells of Cajal; MEG3; apoptosis; nuclear factor kappa-B; oxidative stress.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Overexpression of long non-coding RNA TUG1 alleviates TNF-α-induced inflammatory injury in interstitial cells of Cajal.Eur Rev Med Pharmacol Sci. 2019 Jan;23(1):312-320. doi: 10.26355/eurrev_201901_16778. Eur Rev Med Pharmacol Sci. 2019. Retraction in: Eur Rev Med Pharmacol Sci. 2023 Jul;27(13):5946. doi: 10.26355/eurrev_202307_32942. PMID: 30657572 Retracted.
-
LncRNA MEG3 Participates in Caerulein-Induced Inflammatory Injury in Human Pancreatic Cells via Regulating miR-195-5p/FGFR2 Axis and Inactivating NF-κB Pathway.Inflammation. 2021 Feb;44(1):160-173. doi: 10.1007/s10753-020-01318-6. Inflammation. 2021. PMID: 32856219
-
Downregulation of long noncoding RNA SNHG7 protects against inflammation and apoptosis in Parkinson's disease model by targeting the miR-425-5p/TRAF5/NF-κB axis.J Biochem Mol Toxicol. 2021 Oct;35(10):e22867. doi: 10.1002/jbt.22867. Epub 2021 Aug 8. J Biochem Mol Toxicol. 2021. PMID: 34369042
-
TNF-α inhibits SCF, ghrelin, and substance P expressions through the NF-κB pathway activation in interstitial cells of Cajal.Braz J Med Biol Res. 2018;51(6):e7065. doi: 10.1590/1414-431x20187065. Epub 2018 Apr 23. Braz J Med Biol Res. 2018. PMID: 29694505 Free PMC article.
-
Targeting oxidative stress with natural products: A novel strategy for esophageal cancer therapy.World J Gastrointest Oncol. 2024 Feb 15;16(2):287-299. doi: 10.4251/wjgo.v16.i2.287. World J Gastrointest Oncol. 2024. PMID: 38425393 Free PMC article. Review.
Cited by
-
Harnessing the Anti-Inflammatory Properties of Polyphenols in the Treatment of Inflammatory Bowel Disease.Int J Biol Sci. 2024 Oct 14;20(14):5608-5672. doi: 10.7150/ijbs.98107. eCollection 2024. Int J Biol Sci. 2024. PMID: 39494333 Free PMC article. Review.
-
The long noncoding RNA Meg3 mediates TLR4-induced inflammation in experimental obstructive nephropathy.Clin Sci (Lond). 2023 Mar 15;137(5):317-331. doi: 10.1042/CS20220537. Clin Sci (Lond). 2023. PMID: 36705251 Free PMC article.
-
MEG3 sponges miRNA-376a and YBX1 to regulate angiogenesis in ovarian cancer endothelial cells.Heliyon. 2023 Jan 24;9(2):e13204. doi: 10.1016/j.heliyon.2023.e13204. eCollection 2023 Feb. Heliyon. 2023. PMID: 36747515 Free PMC article.
-
Interstitial cells of Cajal in gastrointestinal inflammatory diseases.J Smooth Muscle Res. 2023;59:1-13. doi: 10.1540/jsmr.59.1. J Smooth Muscle Res. 2023. PMID: 36792171 Free PMC article. Review.
-
Long non-coding RNA maternally expressed gene 3, miR-125a-5p, CXCL13, and NF-kB in patients with immune thrombocytopenia.Genes Immun. 2023 Apr;24(2):108-115. doi: 10.1038/s41435-023-00200-3. Epub 2023 Apr 12. Genes Immun. 2023. PMID: 37045944 Free PMC article.
References
-
- Huizinga JD, Hussain A, Chen JH.. Interstitial cells of Cajal and human colon motility in health and disease[J]. Am J Physiol Gastrointest Liver Physiol. 2021;321(5):G552–g575. - PubMed
-
- Drumm BT, Koh SD, Andersson K-E, et al. Calcium signalling in Cajal-like interstitial cells of the lower urinary tract[J]. Nat Rev Urol. 2014;11(10):555–564. - PubMed
-
- Wishahi M, Mehena AA, Elganzoury H, et al. Telocyte and Cajal cell distribution in renal pelvis, ureteropelvic junction (UPJ), and proximal ureter in normal upper urinary tract and UPJ obstruction: reappraisal of the aetiology of UPJ obstruction[J]. Folia Morphol (Warsz). 2021;80(4):850–856. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
