Terpinen-4-ol inhibits the proliferation and mobility of pancreatic cancer cells by downregulating Rho-associated coiled-coil containing protein kinase 2
- PMID: 35322742
- PMCID: PMC9161900
- DOI: 10.1080/21655979.2022.2054205
Terpinen-4-ol inhibits the proliferation and mobility of pancreatic cancer cells by downregulating Rho-associated coiled-coil containing protein kinase 2
Abstract
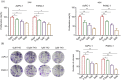
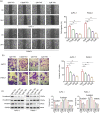
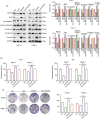
Terpinen-4-ol (T4O), a compound isolated from the seeds of turmeric, has exhibited anti-malignancy, anti-aging, and anti-inflammatory properties in previous studies. However, the specific effects and molecular mechanisms of T4O on pancreatic cancer (PC) cells remain largely unknown. In this study, we demonstrated that T4O markedly suppressed PC cell proliferation and colony formation in vitro and induced apoptosis. Similarly, T4O significantly inhibited the migration and invasion of PC cells in vitro. Through RNA sequencing, 858 differentially expressed genes (DEGs) were identified, which were enriched in the Rhodopsin (RHO)/ Ras homolog family member A (RHOA) signaling pathway. Rho-associated coiled-coil containing protein kinase 2 (ROCK2), a DEG enriched in the RHO/RHOA signaling pathway, was considered as a key target of T4O in PC cells; it was significantly reduced after T4O treatment, highly expressed in PC tissues, and negatively associated with patient outcome. Overexpression of ROCK2 significantly reduced the inhibitory effects of T4O on PC cell proliferation and mobility. Moreover, T4O inhibited cell proliferation in vivo and decreased the Ki-67, cell nuclear antigen, EMT markers, and ROCK2 expression. In conclusion, we consider that T4O can suppress the malignant biological behavior of PC by reducing the expression of ROCK2, thus contributing to PC therapy.
Keywords: Pancreatic cancer; ROCK2; mobility; proliferation; terpinen-4-ol.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
Terpinen-4-ol Induces Ferroptosis of Glioma Cells via Downregulating JUN Proto-Oncogene.Molecules. 2023 Jun 8;28(12):4643. doi: 10.3390/molecules28124643. Molecules. 2023. PMID: 37375197 Free PMC article.
-
Terpinen-4-ol suppresses proliferation and motility of cutaneous squamous cell carcinoma cells by enhancing calpain-2 expression.Oncol Res. 2025 Feb 28;33(3):605-616. doi: 10.32604/or.2024.050661. eCollection 2025. Oncol Res. 2025. PMID: 40109860 Free PMC article.
-
Upregulation of miR-345-5p suppresses cell growth of lung adenocarcinoma by regulating ras homolog family member A (RhoA) and Rho/Rho associated protein kinase (Rho/ROCK) pathway.Chin Med J (Engl). 2021 Oct 14;134(21):2619-2628. doi: 10.1097/CM9.0000000000001804. Chin Med J (Engl). 2021. PMID: 34748526 Free PMC article.
-
Rho-associated coiled-coil kinase (ROCK) signaling and disease.Crit Rev Biochem Mol Biol. 2013 Jul-Aug;48(4):301-16. doi: 10.3109/10409238.2013.786671. Epub 2013 Apr 19. Crit Rev Biochem Mol Biol. 2013. PMID: 23601011 Review.
-
Functions of Rho family of small GTPases and Rho-associated coiled-coil kinases in bone cells during differentiation and mineralization.Biochim Biophys Acta Gen Subj. 2017 May;1861(5 Pt A):1009-1023. doi: 10.1016/j.bbagen.2017.02.005. Epub 2017 Feb 8. Biochim Biophys Acta Gen Subj. 2017. PMID: 28188861 Review.
Cited by
-
Exploring the Therapeutic Potential for Breast Cancer of Phytochemicals and Secondary Metabolites in Marjoram, Thyme, and Persimmon.Metabolites. 2024 Nov 25;14(12):652. doi: 10.3390/metabo14120652. Metabolites. 2024. PMID: 39728433 Free PMC article. Review.
-
Exploring the Anticancer Potential of Origanum majorana Essential Oil Monoterpenes Alone and in Combination against Non-Small Cell Lung Cancer.Nutrients. 2023 Dec 4;15(23):5010. doi: 10.3390/nu15235010. Nutrients. 2023. PMID: 38068868 Free PMC article.
-
Chemical Composition, In Vitro Antitumor Effect, and Toxicity in Zebrafish of the Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae).Biomolecules. 2023 Sep 24;13(10):1439. doi: 10.3390/biom13101439. Biomolecules. 2023. PMID: 37892120 Free PMC article.
-
Ar-turmerone inhibits the proliferation and mobility of glioma by downregulating cathepsin B.Aging (Albany NY). 2023 Sep 26;15(18):9377-9390. doi: 10.18632/aging.204940. Epub 2023 Sep 26. Aging (Albany NY). 2023. PMID: 37768200 Free PMC article.
-
Negative terpinen-4-ol modulate potentially malignant and malignant lingual lesions induced by 4-nitroquinoline-1-oxide in rat model.Naunyn Schmiedebergs Arch Pharmacol. 2022 Nov;395(11):1387-1403. doi: 10.1007/s00210-022-02275-7. Epub 2022 Aug 9. Naunyn Schmiedebergs Arch Pharmacol. 2022. PMID: 35943514
References
-
- Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. Lancet. 2020;395(10242):2008–2020. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
