HIV-1 infection in sickle cell disease and sickle cell trait: role of iron and innate response
- PMID: 35322747
- PMCID: PMC9041812
- DOI: 10.1080/17474086.2022.2054799
HIV-1 infection in sickle cell disease and sickle cell trait: role of iron and innate response
Abstract
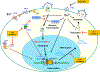
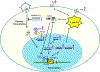
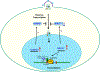
Introduction: Sickle cell disease (SCD), an inherited hemoglobinopathy, affects primarily African Americans in the U.S.A. In addition, about 15% African Americans carry sickle cell trait (SCT). Despite the risk associated with blood transfusions, SCD patients have lower risk of acquiring HIV-1 infection. SCT individuals might also have some protection from HIV-1 infection.
Areas covered: Here, we will review recent and previous studies with the focus on molecular mechanisms that might underlie and contribute to the protection of individuals with SCD and SCT from HIV-1 infection. As both of these conditions predispose to hemolysis, we will focus our discussion on the effects of systemic and intracellular iron on HIV-1 infection and progression. We will also review changes in iron metabolism and activation of innate antiviral responses in SCD and SCT and their effects on HIV-1 infection.
Expert opinion: Previous studies, including ours, showed that SCD might protect from HIV-1 infection. This protection is likely due to the upregulation of complex protein network in response to hemolysis, hypoxia and interferon signaling. These findings are important not only for HIV-1 field but also for SCD cure efforts as antiviral state of SCD patients may adversely affect lentivirus-based gene therapy efforts.
Keywords: HIV-1 infection; Sickle cell disease; anti-viral innate immune response; iron metabolism; sickle cell trait.
Conflict of interest statement
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures
Similar articles
-
Restriction of HIV-1 infection in sickle cell trait.Blood Adv. 2021 Dec 14;5(23):4922-4934. doi: 10.1182/bloodadvances.2021004247. Blood Adv. 2021. PMID: 34496009 Free PMC article.
-
Clinical outcomes of COVID-19 in patients with sickle cell disease and sickle cell trait: A critical appraisal of the literature.Blood Rev. 2022 May;53:100911. doi: 10.1016/j.blre.2021.100911. Epub 2021 Nov 20. Blood Rev. 2022. PMID: 34838342 Free PMC article. Review.
-
Sudden Death in Diabetic Ketoacidosis Complicated by Sickle Cell Trait.Am J Forensic Med Pathol. 2022 Sep 1;43(3):277-281. doi: 10.1097/PAF.0000000000000751. Epub 2022 Feb 9. Am J Forensic Med Pathol. 2022. PMID: 35135968 Review.
-
Blood rheology and vascular function in sickle cell trait and sickle cell disease: From pathophysiological mechanisms to clinical usefulness.Clin Hemorheol Microcirc. 2024;86(1-2):9-27. doi: 10.3233/CH-238122. Clin Hemorheol Microcirc. 2024. PMID: 38073384 Review.
-
COVID-19 in individuals with sickle cell disease/trait compared with other Black individuals.Blood Adv. 2021 Apr 13;5(7):1915-1921. doi: 10.1182/bloodadvances.2020003741. Blood Adv. 2021. PMID: 33792626 Free PMC article.
Cited by
-
Antiviral response and HIV-1 inhibition in sickle cell disease.iScience. 2024 Jan 6;27(2):108813. doi: 10.1016/j.isci.2024.108813. eCollection 2024 Feb 16. iScience. 2024. PMID: 38318349 Free PMC article.
-
Ferroptosis as an emerging target in sickle cell disease.Curr Res Toxicol. 2024 Jun 18;7:100181. doi: 10.1016/j.crtox.2024.100181. eCollection 2024. Curr Res Toxicol. 2024. PMID: 39021403 Free PMC article.
-
Hemoglobinopathies, merozoite surface protein-2 gene polymorphisms, and acquisition of Epstein Barr virus among infants in Western Kenya.BMC Cancer. 2023 Jun 20;23(1):566. doi: 10.1186/s12885-023-11063-2. BMC Cancer. 2023. PMID: 37340364 Free PMC article.
-
Alterations of T Cell Subsets Associated with Sickle Cell Trait.Blood Genom Discov. 2025;9(1):10001. doi: 10.70322/bgd.2025.10001. Epub 2024 Oct 8. Blood Genom Discov. 2025. PMID: 39720621 Free PMC article.
-
Methylation profile of individuals with sickle cell trait.Epigenetics. 2025 Dec;20(1):2539234. doi: 10.1080/15592294.2025.2539234. Epub 2025 Aug 4. Epigenetics. 2025. PMID: 40758858 Free PMC article.
References
-
- Bender MA. Sickle Cell Disease. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews((R)). Seattle (WA)1993. - PubMed
-
- Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nature reviews Disease primers. 2018. Mar 15;4:18010. - PubMed
-
- Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004. Feb 26;350(9):886–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
