The monoacylglycerol acyltransferase pathway contributes to triacylglycerol synthesis in HepG2 cells
- PMID: 35322811
- PMCID: PMC8943211
- DOI: 10.1038/s41598-022-08946-y
The monoacylglycerol acyltransferase pathway contributes to triacylglycerol synthesis in HepG2 cells
Abstract
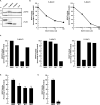
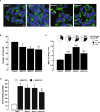
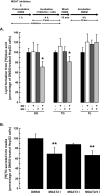
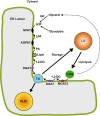
The monoacylglycerol acyltransferase (MGAT) pathway has a well-established role in the small intestine where it facilitates the absorption of dietary fat. In enterocytes, MGAT participates in the resynthesis of triacylglycerol using substrates (monoacylglycerol and fatty acids) generated in the gut lumen from the breakdown of triacylglycerol consumed in the diet. MGAT activity is also present in the liver, but its role in triacylglycerol metabolism in this tissue remains unclear. The predominant MGAT isoforms present in human liver appear to be MGAT2 and MGAT3. The objective of this study was to use selective small molecule inhibitors of MGAT2 and MGAT3 to determine the contributions of these enzymes to triacylglycerol production in liver cells. We found that pharmacological inhibition of either enzyme had no effect on TG mass in HepG2 cells but did alter lipid droplet size and number. Inhibition of MGAT2 did result in decreased DG and TG synthesis and TG secretion. Interestingly, MGAT2 preferentially utilized 2-monoacylglycerol derived from free glycerol and not from exogenously added 2-monoacylglycerol. In contrast, inhibition of MGAT3 had very little effect on TG metabolism in HepG2 cells. Additionally, we demonstrated that the MGAT activity of DGAT1 only makes a minor contribution to TG synthesis in intact HepG2 cells. Our data demonstrated that the MGAT pathway has a role in hepatic lipid metabolism with MGAT2, more so than MGAT3, contributing to TG synthesis and secretion.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Diacylglycerol acyltransferase-2 (DGAT2) and monoacylglycerol acyltransferase-2 (MGAT2) interact to promote triacylglycerol synthesis.J Biol Chem. 2014 Oct 10;289(41):28237-48. doi: 10.1074/jbc.M114.571190. Epub 2014 Aug 27. J Biol Chem. 2014. PMID: 25164810 Free PMC article.
-
Biochemical characterization of human acyl coenzyme A: 2-monoacylglycerol acyltransferase-3 (MGAT3).Biochem Biophys Res Commun. 2016 Jul 1;475(3):264-70. doi: 10.1016/j.bbrc.2016.05.071. Epub 2016 May 13. Biochem Biophys Res Commun. 2016. PMID: 27184406
-
Catalytic properties of MGAT3, a putative triacylgycerol synthase.J Lipid Res. 2007 Mar;48(3):583-91. doi: 10.1194/jlr.M600331-JLR200. Epub 2006 Dec 14. J Lipid Res. 2007. PMID: 17170429
-
Mechanisms of intestinal triacylglycerol synthesis.Biochim Biophys Acta Mol Cell Biol Lipids. 2022 Jun;1867(6):159151. doi: 10.1016/j.bbalip.2022.159151. Epub 2022 Mar 14. Biochim Biophys Acta Mol Cell Biol Lipids. 2022. PMID: 35296424 Review.
-
Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism.Am J Physiol Endocrinol Metab. 2009 Jul;297(1):E10-8. doi: 10.1152/ajpendo.90949.2008. Epub 2008 Dec 30. Am J Physiol Endocrinol Metab. 2009. PMID: 19116371 Free PMC article. Review.
Cited by
-
Leonurine Inhibits Hepatic Lipid Synthesis to Ameliorate NAFLD via the ADRA1a/AMPK/SCD1 Axis.Int J Mol Sci. 2024 Oct 9;25(19):10855. doi: 10.3390/ijms251910855. Int J Mol Sci. 2024. PMID: 39409181 Free PMC article.
-
Serotonin Transporter Deficiency Induces Metabolic Alterations in the Ileal Mucosa.Int J Mol Sci. 2024 Apr 18;25(8):4459. doi: 10.3390/ijms25084459. Int J Mol Sci. 2024. PMID: 38674044 Free PMC article.
-
Integrated Lipidomics and Transcriptomics Analyses Reveal Key Regulators of Fat Deposition in Different Adipose Tissues of Geese (Anser cygnoides).Animals (Basel). 2024 Jul 5;14(13):1990. doi: 10.3390/ani14131990. Animals (Basel). 2024. PMID: 38998104 Free PMC article.
-
Identification of genomic regions associated with fatty acid metabolism across blood, liver, backfat and muscle in pigs.Genet Sel Evol. 2024 Sep 26;56(1):66. doi: 10.1186/s12711-024-00933-3. Genet Sel Evol. 2024. PMID: 39327557 Free PMC article.
-
Host Cell Response to Rotavirus Infection with Emphasis on Virus-Glycan Interactions, Cholesterol Metabolism, and Innate Immunity.Viruses. 2023 Jun 21;15(7):1406. doi: 10.3390/v15071406. Viruses. 2023. PMID: 37515094 Free PMC article.
References
-
- Enjoji, M., Kohjima, M. & Nakamuta, M. in The Liver in Systemic Diseases (ed Hiromasa Ohira) 105–122 (Springer Japan, 2016).
-
- Brindley, D. N. in Biochemistry of Lipids, Lipoproteins and Membranes (eds D E Vance & J E Vance) 171–203 (Elsevier, 1991).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

