Type 2 diabetes and the clinically normal pulp: An in vitro study
- PMID: 35322881
- PMCID: PMC9324782
- DOI: 10.1111/iej.13732
Type 2 diabetes and the clinically normal pulp: An in vitro study
Abstract
Aim: The aim of this study was to investigate the effect of type 2 diabetes (T2D) on clinically normal dental pulp tissue by using special stains and immunohistochemistry (IHC) to determine the morphology of the coronal pulp and distribution of immune markers in non-T2D and T2D groups.
Methodology: Ethics approval for this in vitro pilot study was obtained from the University of Otago Human Ethics Committee (16/069). Twenty extracted permanent molar teeth diagnosed as having clinically normal pulp status were collected. Ten teeth were from participants with well-controlled T2D and ten from participants without diabetes (non-T2D). Each tooth was sectioned transversely at the cemento-enamel junction before the crowns were decalcified and embedded in paraffin. Sections were stained with haematoxylin and eosin, Massons trichrome, and van Gieson stains for histological and morphological evaluation. IHC using anti-CD4, anti-CD68 and anti-CD83 and anti-IL1β, anti-IL6, anti-IL17, anti-TNF-α, anti-TLR2, anti-TLR4 and anti-FOXP3 identified proteins of interest. Qualitative and semi-quantitative analyses evaluated the morphology of the dental pulp and protein expression. Data analyses were performed with GraphPad Prism, using Student's t-test and multiple regression using SPSS at p < .05.
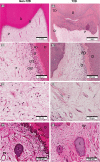
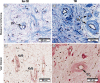
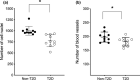
Results: Special stains demonstrated morphological differences in the T2D dental pulp compared with non-T2D. Qualitative analysis indicated that the pulp in the T2D samples was consistently less cellular, less vascular, showed evidence of thickened blood vessel walls, increased pulp calcification and collagen deposition. Semi-quantitative analysis of IHC samples showed the T2D pulp had significantly increased expression of macrophage and dendritic cell markers CD68 (p < .001) and CD83 (p = .04), and there was significantly greater expression of inflammatory cytokines IL1β (p = .01), IL6 (p < .0001), IL17 (p < .0001) and TNF-α (p = .01). T2D samples showed a significant increase in markers of innate inflammation, TLR2 (p < .001) and TLR4 (p < .001) and decreased expression of regulatory T-cell marker, FOXP3 (p = .01). Multiple regression showed that age-corrected differences were statistically significant.
Conclusion: Preliminary findings suggest that T2D may exert a similar response in the pulp to complications in other body sites. Hyperglycaemia is associated with changes in the morphology of the clinically normal dental pulp with altered immune cell and cytokine expression.
Keywords: dental pulp; histomorphology; immune response; immunohistochemistry; type 2 diabetes.
© 2022 The Authors. International Endodontic Journal published by John Wiley & Sons Ltd on behalf of British Endodontic Society.
Conflict of interest statement
No conflict of interest.
Figures

References
-
- Abdel‐Moneim, A. , Bakery, H.H. & Allam, G. (2018) The potential pathogenic role of IL‐17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomedicine & Pharmacotherapy, 101, 287–292. - PubMed
-
- Amatyakul, S. , Chakraphan, D. , Chotpaibulpan, S. & Patumraj, S. (2003) The effect of long‐term supplementation of vitamin C on pulpal blood flow in streptozotocin‐induced diabetic rats. Clinical Hemorheology and Microcirculation, 29, 313–319. - PubMed
-
- American Association of Endodontists . (2009) AAE Consensus conference recommended diagnostic terminology. Journal of Endodontics, 35, 1634.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

