Cardiovascular drugs and COVID-19 clinical outcomes: a systematic review and meta-analysis of randomized controlled trials
- PMID: 35322889
- PMCID: PMC9111446
- DOI: 10.1111/bcp.15331
Cardiovascular drugs and COVID-19 clinical outcomes: a systematic review and meta-analysis of randomized controlled trials
Abstract
Aims: To update our previously reported systematic review and meta-analysis of observational studies on cardiovascular drug exposure and COVID-19 clinical outcomes by focusing on newly published randomized controlled trials (RCTs).
Methods: More than 500 databases were searched between 1 November 2020 and 2 October 2021 to identify RCTs that were published after our baseline review. One reviewer extracted data with other reviewers verifying the extracted data for accuracy and completeness.
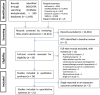
Results: After screening 22 414 records, we included 24 and 21 RCTs in the qualitative and quantitative syntheses, respectively. The most investigated drug classes were angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blocker (ARBs) and anticoagulants, investigated by 10 and 11 studies respectively. In meta-analyses, ACEI/ARBs did not affect hospitalization length (mean difference -0.42, 95% confidence interval [CI] -1.83; 0.98 d, n = 1183), COVID-19 severity (risk ratio/RR 0.90, 95% CI 0.71; 1.15, n = 1661) or mortality (risk ratio [RR] 0.92, 95% CI 0.58; 1.47, n = 1646). Therapeutic anticoagulation also had no effect (hospitalization length mean difference -0.29, 95% CI -1.13 to 0.56 d, n = 1449; severity RR 0.86, 95% CI 0.70; 1.04, n = 2696; and, mortality RR 0.93, 95% CI 0.77; 1.13, n = 5689). Other investigated drug classes were antiplatelets (aspirin, 2 trials), antithrombotics (sulodexide, 1 trial), calcium channel blockers (amlodipine, 1 trial) and lipid-modifying drugs (atorvastatin, 1 trial).
Conclusion: Moderate- to high-certainty RCT evidence suggests that cardiovascular drugs such as ACEIs/ARBs are not associated with poor COVID-19 outcomes, and should therefore not be discontinued. These cardiovascular drugs should also not be initiated to treat or prevent COVID-19 unless they are needed for an underlying currently approved therapeutic indication.
Keywords: COVID-19; RCTs; cardiovascular drugs; living systematic review; meta-analysis.
© 2022 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
M.P. has received partnership funding for the following: MRC Clinical Pharmacology Training Scheme (co‐funded by MRC and Roche, UCB, Eli Lilly and Novartis); a PhD studentship jointly funded by EPSRC and Astra Zeneca; and grant funding from Vistagen Therapeutics. He has also unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol‐Myers Squibb and UCB. He has developed an HLA genotyping panel with MC Diagnostics, but does not benefit financially from this. He is part of the IMI Consortium ARDAT (
Figures


References
-
- World Health Organisation . WHO Director‐General's opening remarks at the media briefing on COVID‐19‐11 March 2020. World Health Organisation. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re.... Published 2020. Accessed 6 June, 2020.
-
- Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID‐19 and Cardiovascular Disease. Circulation. 2020;141(20):1648‐1655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

