Tumorigenic transformation of human prostatic epithelial cell line RWPE-1 by growth hormone-releasing hormone (GHRH)
- PMID: 35322894
- PMCID: PMC9310601
- DOI: 10.1002/pros.24339
Tumorigenic transformation of human prostatic epithelial cell line RWPE-1 by growth hormone-releasing hormone (GHRH)
Abstract
Background: Growth hormone-releasing hormone (GHRH) and its receptors have been implicated in the progression of various tumors. In this study, we analyzed the carcinogenetic potential of exposure to GHRH of a nontumor human prostate epithelial cell line (RWPE-1) as well as its transforming effect in a xenograft model.
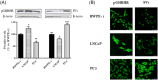
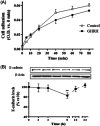
Methods: We performed cell viability, cell proliferation, adhesion and migration assays. In addition, metalloprotease (MMP)-2 activity by means gelatin zymography, GHRH-R subcellular location using confocal immunofluorescence microscopy and vascular endothelial growth factor (VEGF) levels by enzyme-linked immunoassay were assessed. Besides, we developed an in vivo model in order vivo model to determine the role of GHRH on tumorigenic transformation of RWPE-1 cells.
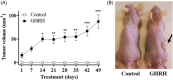
Results: In cell cultures, we observed development of a migratory phenotype consistent with the gelatinolytic activity of MMP-2, expression of VEGF, as well as E-cadherin-mediated cell-cell adhesion and increased cell motility. Treatment with 0.1 µM GHRH for 24 h significantly increased cell viability and cell proliferation. Similar effects of GHRH were seen in RWPE-1 tumors developed by subcutaneous injection of GHRH-treated cells in athymic nude mice, 49 days after inoculation.
Conclusions: Thus, GHRH appears to act as a cytokine in the transformation of RWPE-1 cells by mechanisms that likely involve epithelial-mesenchymal transition, thus reinforcing the role of GHRH in tumorigenesis of prostate.
Keywords: GHRH; RWPE-1 cells; epithelial-mesenchymal transition; prostate cancer; tumorigenesis.
© 2022 The Authors. The Prostate published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Singh VK, Pal R, Srivastava P, Misra G, Shukla Y, Sharma PK. Exposure of androgen mimicking environmental chemicals enhances proliferation of prostate cancer (LNCaP) cells by inducing AR expression and epigenetic modifications. Environ Pollut. 2021;272:116397. 10.1016/j.envpol.2020.116397 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

