Towards Next-Generation Sequencing (NGS)-Based Newborn Screening: A Technical Study to Prepare for the Challenges Ahead
- PMID: 35323196
- PMCID: PMC8949100
- DOI: 10.3390/ijns8010017
Towards Next-Generation Sequencing (NGS)-Based Newborn Screening: A Technical Study to Prepare for the Challenges Ahead
Abstract
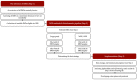
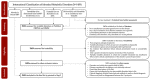
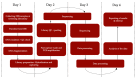
Newborn screening (NBS) aims to identify neonates with severe conditions for whom immediate treatment is required. Currently, a biochemistry-first approach is used to identify these disorders, which are predominantly inherited meta1bolic disorders (IMD). Next-generation sequencing (NGS) is expected to have some advantages over the current approach, for example the ability to detect IMDs that meet all screening criteria but lack an identifiable biochemical footprint. We have now designed a technical study to explore the use of NGS techniques as a first-tier approach in NBS. Here, we describe the aim and set-up of the NGS-first for the NBS (NGSf4NBS) project, which will proceed in three steps. In Step 1, we will identify IMDs eligible for NGS-first testing, based on treatability. In Step 2, we will investigate the feasibility, limitations and comparability of different technical NGS approaches and analysis workflows for NBS, eventually aiming to develop a rapid NGS-based workflow. Finally, in Step 3, we will prepare for the incorporation of this workflow into the existing Dutch NBS program and propose a protocol for referral of a child after a positive NGS test result. The results of this study will be the basis for an additional analytical route within NBS that will be further studied for its applicability within the NBS program, e.g., regarding the ethical, legal, financial and social implications.
Keywords: dried blood spot; first-tier; heel prick; inborn errors of metabolism; inherited metabolic disorders; newborn screening; next-generation sequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Blom M., Bredius R.G., Weijman G., Dekkers E.H., Kemper E.A., den Akker-van Marle V., Elske M., Van der Ploeg C.P., Van der Burg M., Schielen P.C. Introducing Newborn Screening for Severe Combined Immunodeficiency (SCID) in the Dutch Neonatal Screening Program. Int. J. Neonatal Screen. 2018;4:40. doi: 10.3390/ijns4040040. - DOI - PMC - PubMed
-
- van der Meij K.R., Sistermans E.A., Macville M.V., Stevens S.J., Bax C.J., Bekker M.N., Bilardo C.M., Boon E.M., Boter M., Diderich K.E., et al. TRIDENT-2: National Implementation of Genome-wide Non-invasive Prenatal Testing as a First-Tier Screening Test in the Netherlands. Am. J. Hum. Genet. 2019;105:1091–1101. doi: 10.1016/j.ajhg.2019.10.005. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

