Combined Effects of Fludarabine and Interferon Alpha on Autophagy Regulation Define the Phase of Cell Survival and Promotes Responses in LLC-MK2 and K562 Cells
- PMID: 35323219
- PMCID: PMC8950195
- DOI: 10.3390/medsci10010020
Combined Effects of Fludarabine and Interferon Alpha on Autophagy Regulation Define the Phase of Cell Survival and Promotes Responses in LLC-MK2 and K562 Cells
Abstract
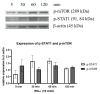
Autophagy is a known mechanism of cells under internal stress that regulates cellular function via internal protein recycling and the cleaning up of debris, leading to healthy live cells. However, the stimulation of autophagy by external factors such as chemical compounds or viral infection mostly tends to induce apoptosis/cell death. This study hypothesizes that manipulation of the autophagy mechanism to the pro-cell survival and/or decreased pro-viral niche can be a strategy for effective antiviral and anticancer treatment. Cells susceptible to viral infection, namely LLC-MK2, normal monkey epithelium, and K562, human immune-related lymphocyte, which is also a cancer cell line, were treated with fludarabine nucleoside analog (Fdb), interferon alpha (IFN-α), and a combination of Fdb and IFN-α, and then were evaluated for signs of adaptive autophagy and STAT1 antiviral signaling by Western blotting and immunolabeling assays. The results showed that the low concentration of Fdb was able to activate an autophagy response in both cell types, as demonstrated by the intense immunostaining of LC3B foci in the autophagosomes of living cells. Treatment with IFN-α (10 U/mL) showed no alteration in the initiator of mTOR autophagy but dramatically increased the intracellular STAT1 signaling molecules in both cell types. Although in the combined Fdb and IFN-α treatment, both LLC-MK2 and K562 cells showed only slight changes in the autophagy-responsive proteins p-mTOR and LC3B, an adaptive autophagy event was clearly shown in the autophagosome of the LLC-MK2 cell, suggesting the survival phase of the normal cell. The combined effect of Fdb and IFN-α treatment on the antiviral response was identified by the level of activation of the STAT1 antiviral marker. Significantly, the adaptive autophagy mediated by Fdb was able to suppress the IFN-α-mediated pSTAT1 signaling in both cell types to a level that is appropriate for cellular function. It is concluded that the administration of an appropriate dose of Fdb and IFN-α in combination is beneficial for the treatment of some types of cancer and viral infection.
Keywords: K562; LLC-MK2; STAT1; autophagy; fludarabine; interferon-alpha.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Interferon alpha induced intrahepatic pSTAT1 inversely correlate with serum HCV RNA levels in chronic HCV infection.Exp Mol Pathol. 2014 Feb;96(1):36-41. doi: 10.1016/j.yexmp.2013.10.016. Epub 2013 Nov 8. Exp Mol Pathol. 2014. PMID: 24211829 Free PMC article.
-
The Chinese herbal prescription JZ-1 induces autophagy to protect against herpes simplex Virus-2 in human vaginal epithelial cells by inhibiting the PI3K/Akt/mTOR pathway.J Ethnopharmacol. 2020 May 23;254:112611. doi: 10.1016/j.jep.2020.112611. Epub 2020 Feb 20. J Ethnopharmacol. 2020. PMID: 32088246 Free PMC article.
-
Screening interferon antagonists from accessory proteins encoded by P gene for immune escape of Caprine parainfluenza virus 3.Vet Microbiol. 2021 Mar;254:108980. doi: 10.1016/j.vetmic.2021.108980. Epub 2021 Jan 6. Vet Microbiol. 2021. PMID: 33445054
-
HIV-1 Promotes the Degradation of Components of the Type 1 IFN JAK/STAT Pathway and Blocks Anti-viral ISG Induction.EBioMedicine. 2018 Apr;30:203-216. doi: 10.1016/j.ebiom.2018.03.006. Epub 2018 Mar 9. EBioMedicine. 2018. PMID: 29580840 Free PMC article.
-
Interferon-α and its effects on cancer cell apoptosis.Oncol Lett. 2022 May 30;24(1):235. doi: 10.3892/ol.2022.13355. eCollection 2022 Jul. Oncol Lett. 2022. PMID: 35720476 Free PMC article. Review.
References
-
- Yan X., Zhou R., Ma Z. Autophagy—Cell Survival and Death. In: Qin Z.-H., editor. Autophagy: Biology and Diseases: Basic Science. Springer; Singapore: 2019. pp. 667–696. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

