Synthesis and Characterization of Conjugated Hyaluronic Acids. Application to Stability Studies of Chitosan-Hyaluronic Acid Nanogels Based on Fluorescence Resonance Energy Transfer
- PMID: 35323295
- PMCID: PMC8949952
- DOI: 10.3390/gels8030182
Synthesis and Characterization of Conjugated Hyaluronic Acids. Application to Stability Studies of Chitosan-Hyaluronic Acid Nanogels Based on Fluorescence Resonance Energy Transfer
Abstract
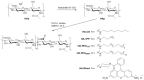
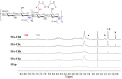
Hyaluronic acid (HA) was functionalized with a series of amino synthons (octylamine, polyethylene glycol amine, trifluoropropyl amine, rhodamine). Sodium hyaluronate (HAs) was first converted into its protonated form (HAp) and the reaction was conducted in DMSO by varying the initial ratio (-NH2 (synthon)/COOH (HAp)). HA derivatives were characterized by a combination of techniques (FTIR, 1H NMR, 1D diffusion-filtered 19F NMR, DOSY experiments), and degrees of substitution (DSHA) varying from 0.3% to 47% were determined, according to the grafted synthon. Nanohydrogels were then obtained by ionic gelation between functionalized hyaluronic acids and chitosan (CS) and tripolyphosphate (TPP) as a cross-linker. Nanohydrogels for which HA and CS were respectively labeled by rhodamine and fluorescein which are a fluorescent donor-acceptor pair were subjected to FRET experiments to evaluate the stability of these nano-assemblies.
Keywords: fluorinated and fluorescent HA conjugates–hyaluronic acid degree of substitution–diffusion ordered spectroscopy (DOSY)–1D diffusion-filtered 19F NMR–atomic force microscopy–FRET experiments–hyaluronidase–nanohydrogel stability; nanohydrogels–hyaluronic acid–HA-mPEG2000.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gallo N., Nasser H., Salvatore L., Natali M.L., Campa L., Mahmoud M., Capobianco L., Sannino A., Madaghiel M. Hyaluronic acid for advanced therapies: Promises and challenges. Eur. Polym. J. 2019;117:134–147. doi: 10.1016/j.eurpolymj.2019.05.007. - DOI
-
- Khan W., Abtew E., Modani S., Domb A.J. Polysaccharide based nanoparticles. Isr. J. Chem. 2018;58:1315–1329. doi: 10.1002/ijch.201800051. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

