Immunocapture Magnetic Beads Enhanced the LAMP-CRISPR/Cas12a Method for the Sensitive, Specific, and Visual Detection of Campylobacter jejuni
- PMID: 35323424
- PMCID: PMC8946501
- DOI: 10.3390/bios12030154
Immunocapture Magnetic Beads Enhanced the LAMP-CRISPR/Cas12a Method for the Sensitive, Specific, and Visual Detection of Campylobacter jejuni
Abstract
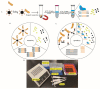
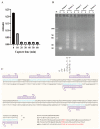
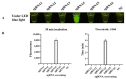
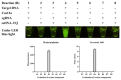
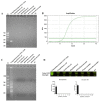
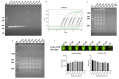
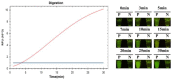
Campylobacter jejuni is one of the most important causes of food-borne infectious disease, and poses challenges to food safety and public health. Establishing a rapid, accurate, sensitive, and simple detection method for C. jejuni enables early diagnosis, early intervention, and prevention of pathogen transmission. In this study, an immunocapture magnetic bead (ICB)-enhanced loop-mediated isothermal amplification (LAMP) CRISPR/Cas12a method (ICB-LAMP-CRISPR/Cas12a) was developed for the rapid and visual detection of C. jejuni. Using the ICB-LAMP-CRISPR/Cas12a method, C. jejuni was first captured by ICB, and the bacterial genomic DNA was then released by heating and used in the LAMP reaction. After the LAMP reaction, LAMP products were mixed and detected by the CRISPR/Cas12a cleavage mixture. This ICB-LAMP-CRISPR/Cas12a method could detect a minimum of 8 CFU/mL of C. jejuni within 70 min. Additionally, the method was performed in a closed tube in addition to ICB capture, which eliminates the need to separate preamplification and transfer of amplified products to avoid aerosol pollution. The ICB-LAMP-CRISPR/Cas12a method was further validated by testing 31 C. jejuni-positive fecal samples from different layer farms. This method is an all-in-one, simple, rapid, ultrasensitive, ultraspecific, visual detection method for instrument-free diagnosis of C. jejuni, and has wide application potential in future work.
Keywords: Campylobacter jejuni; ICB-LAMP-CRISPR/Cas12a; food-borne pathogens; point of care; visual detection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Rapid and Sensitive Detection of Verticillium dahliae from Soil Using LAMP-CRISPR/Cas12a Technology.Int J Mol Sci. 2024 May 10;25(10):5185. doi: 10.3390/ijms25105185. Int J Mol Sci. 2024. PMID: 38791224 Free PMC article.
-
Enhanced detection of Listeria monocytogenes using tetraethylenepentamine-functionalized magnetic nanoparticles and LAMP-CRISPR/Cas12a-based biosensor.Anal Chim Acta. 2023 Nov 15;1281:341905. doi: 10.1016/j.aca.2023.341905. Epub 2023 Oct 11. Anal Chim Acta. 2023. PMID: 38783743
-
CRISPR/Cas12a-Enhanced Loop-Mediated Isothermal Amplification for the Visual Detection of Shigella flexneri.Front Bioeng Biotechnol. 2022 Feb 21;10:845688. doi: 10.3389/fbioe.2022.845688. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35265606 Free PMC article.
-
M-CDC: Magnetic pull-down-assisted colorimetric method based on the CRISPR/Cas12a system.Methods. 2022 Jul;203:259-267. doi: 10.1016/j.ymeth.2021.11.009. Epub 2021 Nov 20. Methods. 2022. PMID: 34813932 Review.
-
CRISPR/Cas12a-based technology: A powerful tool for biosensing in food safety.Trends Food Sci Technol. 2022 Apr;122:211-222. doi: 10.1016/j.tifs.2022.02.030. Epub 2022 Mar 1. Trends Food Sci Technol. 2022. PMID: 35250172 Free PMC article. Review.
Cited by
-
Rapid and Sensitive Detection of Verticillium dahliae from Soil Using LAMP-CRISPR/Cas12a Technology.Int J Mol Sci. 2024 May 10;25(10):5185. doi: 10.3390/ijms25105185. Int J Mol Sci. 2024. PMID: 38791224 Free PMC article.
-
Research progress of CRISPR-based biosensors and bioassays for molecular diagnosis.Front Bioeng Biotechnol. 2022 Sep 16;10:986233. doi: 10.3389/fbioe.2022.986233. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36185462 Free PMC article. Review.
-
PathCrisp: an innovative molecular diagnostic tool for early detection of NDM-resistant infections.Sci Rep. 2025 Jan 2;15(1):490. doi: 10.1038/s41598-024-84832-z. Sci Rep. 2025. PMID: 39747369 Free PMC article.
-
β-Cyclodextrin-Stabilized Silver Nanoparticle Production Combined with Loop-Mediated Isothermal Amplification for the Visual Detection of Contagious Pathogens.Micromachines (Basel). 2024 Mar 12;15(3):378. doi: 10.3390/mi15030378. Micromachines (Basel). 2024. PMID: 38542625 Free PMC article.
-
Comparison of Biosensing Methods Based on Different Isothermal Amplification Strategies: A Case Study with Erwinia amylovora.Biosensors (Basel). 2022 Dec 15;12(12):1174. doi: 10.3390/bios12121174. Biosensors (Basel). 2022. PMID: 36551141 Free PMC article.
References
-
- World Health Organization Campylobacter. [(accessed on 1 October 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter.
-
- Ewers E.C., Anisowicz S.K., Ferguson T.M., Seronello S.E., Barnhill J.C., Lustik M.B., Agee W., 3rd, Washington M.A., Nahid M.A., Burnett M.W., et al. Antibiotic resistance, molecular characterizations, and clinical manifestations of Campylobacteriosis at a military medical center in Hawaii from 2012–2016: A retrospective analysis. Sci. Rep. 2018;8:11736. doi: 10.1038/s41598-018-29461-z. - DOI - PMC - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

