Preparation, Identification, Molecular Docking Study and Protective Function on HUVECs of Novel ACE Inhibitory Peptides from Protein Hydrolysate of Skipjack Tuna Muscle
- PMID: 35323475
- PMCID: PMC8954214
- DOI: 10.3390/md20030176
Preparation, Identification, Molecular Docking Study and Protective Function on HUVECs of Novel ACE Inhibitory Peptides from Protein Hydrolysate of Skipjack Tuna Muscle
Abstract
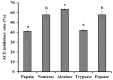
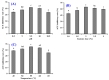
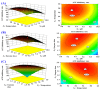
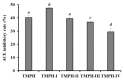
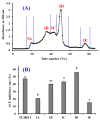
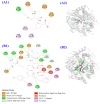
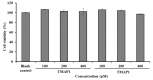
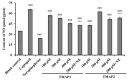
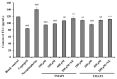
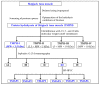
To prepare bioactive peptides with high angiotensin-I-converting enzyme (ACE)-inhibitory (ACEi) activity, Alcalase was selected from five kinds of protease for hydrolyzing Skipjack tuna (Katsuwonus pelamis) muscle, and its best hydrolysis conditions were optimized using single factor and response surface experiments. Then, the high ACEi protein hydrolysate (TMPH) of skipjack tuna muscle was prepared using Alcalase under the optimum conditions of enzyme dose 2.3%, enzymolysis temperature 56.2 °C, and pH 9.4, and its ACEi activity reached 72.71% at 1.0 mg/mL. Subsequently, six novel ACEi peptides were prepared from TMPH using ultrafiltration and chromatography methods and were identified as Ser-Pro (SP), Val-Asp-Arg-Tyr-Phe (VDRYF), Val-His-Gly-Val-Val (VHGVV), Tyr-Glu (YE), Phe-Glu-Met (FEM), and Phe-Trp-Arg-Val (FWRV), with molecular weights of 202.3, 698.9, 509.7, 310.4, 425.6, and 606.8 Da, respectively. SP and VDRYF displayed noticeable ACEi activity, with IC50 values of 0.06 ± 0.01 and 0.28 ± 0.03 mg/mL, respectively. Molecular docking analysis illustrated that the high ACEi activity of SP and VDRYF was attributed to effective interaction with the active sites/pockets of ACE by hydrogen bonding, electrostatic force, and hydrophobic interaction. Furthermore, SP and VDRYF could significantly up-regulate nitric oxide (NO) production and down-regulate endothelin-1 (ET-1) secretion in HUVECs after 24 h treatment, but also abolish the negative effect of 0.5 μM norepinephrine (NE) on the generation of NO and ET-1. Therefore, ACEi peptides derived from skipjack tuna (K. pelamis) muscle, especially SP and VDRYF, are beneficial components for functional food against hypertension and cardiovascular diseases.
Keywords: angiotensin-I-converting enzyme (ACE) peptide; endothelin-1 (ET-1); molecular docking; nitric oxide (NO); skipjack tuna (Katsuwonus pelamis) muscle.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Novel angiotensin-converting enzyme inhibitory peptides from tuna byproducts-milts: Preparation, characterization, molecular docking study, and antioxidant function on H2O2-damaged human umbilical vein endothelial cells.Front Nutr. 2022 Jul 22;9:957778. doi: 10.3389/fnut.2022.957778. eCollection 2022. Front Nutr. 2022. PMID: 35938100 Free PMC article.
-
Preparation, Characterization, and Cytoprotective Effects on HUVECs of Fourteen Novel Angiotensin-I-Converting Enzyme Inhibitory Peptides From Protein Hydrolysate of Tuna Processing By-Products.Front Nutr. 2022 Apr 14;9:868681. doi: 10.3389/fnut.2022.868681. eCollection 2022. Front Nutr. 2022. PMID: 35495901 Free PMC article.
-
Production, identification, in silico analysis, and cytoprotection on H2O2-induced HUVECs of novel angiotensin-I-converting enzyme inhibitory peptides from Skipjack tuna roes.Front Nutr. 2023 Jul 12;10:1197382. doi: 10.3389/fnut.2023.1197382. eCollection 2023. Front Nutr. 2023. PMID: 37502715 Free PMC article.
-
Enzymatic Preparation of Bioactive Peptides Exhibiting ACE Inhibitory Activity from Soybean and Velvet Bean: A Systematic Review.Molecules. 2021 Jun 23;26(13):3822. doi: 10.3390/molecules26133822. Molecules. 2021. PMID: 34201554 Free PMC article.
-
Molecular interactions, bioavailability, and cellular mechanisms of angiotensin-converting enzyme inhibitory peptides.J Food Biochem. 2019 Jan;43(1):e12572. doi: 10.1111/jfbc.12572. Epub 2018 Aug 22. J Food Biochem. 2019. PMID: 31353484 Review.
Cited by
-
Bioactive peptides of marine organisms: Roles in the reduction and control of cardiovascular diseases.Food Sci Nutr. 2024 Apr 23;12(8):5271-5284. doi: 10.1002/fsn3.4183. eCollection 2024 Aug. Food Sci Nutr. 2024. PMID: 39139935 Free PMC article. Review.
-
A review on the processing of functional proteins or peptides derived from fish by-products and their industrial applications.Heliyon. 2023 Mar 1;9(3):e14188. doi: 10.1016/j.heliyon.2023.e14188. eCollection 2023 Mar. Heliyon. 2023. PMID: 36938382 Free PMC article. Review.
-
Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, pH, and Simulated Gastrointestinal Digestion Treatments.Mar Drugs. 2022 Sep 30;20(10):626. doi: 10.3390/md20100626. Mar Drugs. 2022. PMID: 36286450 Free PMC article.
-
Antioxidant Peptides from Monkfish Swim Bladders: Ameliorating NAFLD In Vitro by Suppressing Lipid Accumulation and Oxidative Stress via Regulating AMPK/Nrf2 Pathway.Mar Drugs. 2023 Jun 16;21(6):360. doi: 10.3390/md21060360. Mar Drugs. 2023. PMID: 37367685 Free PMC article.
-
Bioactive Peptides from Marine Organisms.Protein Pept Lett. 2024;31(8):569-585. doi: 10.2174/0109298665329840240816062134. Protein Pept Lett. 2024. PMID: 39253911 Review.
References
-
- Liu X., Li F., Zheng Z., Li G., Zhou H., Zhang T., Tang Y., Qin W. Association of morning hypertension with chronic kidney disease progression and cardiovascular events in patients with chronic kidney disease and hypertension. Nutr. Metab. Cardiovas. 2022;12:021. doi: 10.1016/j.numecd.2021.12.021. - DOI - PubMed
-
- Ning D.S., Ma J., Peng Y.M., Li Y., Chen Y.T., Li S.X., Liu Z., Li Y.Q., Zhang Y.X., Jian Y.P., et al. Apolipoprotein A-I mimetic peptide inhibits atherosclerosis by increasing tetrahydrobiopterin via regulation of GTP-cyclohydrolase 1 and reducing uncoupled endothelial nitric oxide synthase activity. Atherosclerosis. 2021;328:83–91. doi: 10.1016/j.atherosclerosis.2021.05.019. - DOI - PubMed
-
- Abdelhedi O., Nasri M. Basic and recent advances in marine antihypertensive peptides: Production, structure-activity relationship and bioavailability. Trends Food Sci. Technol. 2019;88:43–557. doi: 10.1016/j.tifs.2019.04.002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

