Current Trends and New Challenges in Marine Phycotoxins
- PMID: 35323497
- PMCID: PMC8950113
- DOI: 10.3390/md20030198
Current Trends and New Challenges in Marine Phycotoxins
Abstract
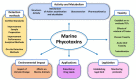
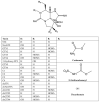
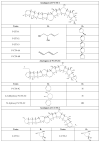
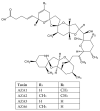
Marine phycotoxins are a multiplicity of bioactive compounds which are produced by microalgae and bioaccumulate in the marine food web. Phycotoxins affect the ecosystem, pose a threat to human health, and have important economic effects on aquaculture and tourism worldwide. However, human health and food safety have been the primary concerns when considering the impacts of phycotoxins. Phycotoxins toxicity information, often used to set regulatory limits for these toxins in shellfish, lacks traceability of toxicity values highlighting the need for predefined toxicological criteria. Toxicity data together with adequate detection methods for monitoring procedures are crucial to protect human health. However, despite technological advances, there are still methodological uncertainties and high demand for universal phycotoxin detectors. This review focuses on these topics, including uncertainties of climate change, providing an overview of the current information as well as future perspectives.
Keywords: detection methods; mechanism of action; phycotoxin; therapeutic application; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Botana L.M., Louzao M.C., Vilariño N. Climate Change and Marine and Freshwater Toxins. 2nd ed. De Gruyter; Berlin, Germany: 2021. p. 661.
-
- EFSA Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on Marine Biotoxins in Shellfish—Saxitoxin Group. EFSA J. 2009;1019:1–76.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

