Crystallography of Contemporary Contact Insecticides
- PMID: 35323590
- PMCID: PMC8949367
- DOI: 10.3390/insects13030292
Crystallography of Contemporary Contact Insecticides
Abstract
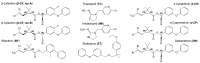
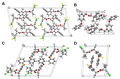
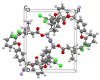
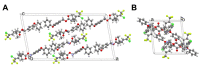
The active forms of contact insecticides used for combatting mosquito-borne infectious diseases are typically crystalline solids. Numerous molecular crystals are polymorphic, crystallizing in several solid forms characterized by different physicochemical properties, including bioavailability. Our laboratory recently found that the activity of crystalline contact insecticides is inversely dependent on the thermodynamic stability of their polymorphs, suggesting that efficacy can be enhanced by the manipulation of the solid-state structure. This paper argues that crystallography should be central to the development of contact insecticides, particularly because their efficacy continues to be compromised by insecticide resistance, especially among Anopheles mosquito populations that spread malaria. Although insecticidal compounds with new modes of action have been introduced to overcome resistance, new insecticides are expensive to develop and implement. The repurposing of existing chemical agents in metastable, more active crystalline forms provides an inexpensive and efficient method for 'evergreening' compounds whose risks are already well-established. We report herein seven new single-crystal structures of insecticides used for controlling infectious disease vectors. The structures reported herein include pyrethroid insecticides recommended by the WHO for indoor residual spraying (IRS)-bifenthrin, β-cyfluthrin, etofenprox, α-cypermethrin, and λ-cyhalothrin as well as the neonicotinoid insecticide thiacloprid.
Keywords: bifenthrin; deltamethrin; etofenprox; imidacloprid; malaria; mosquitoes; thiacloprid; α-cypermethrin; β-cyfluthrin; λ-cyhalothrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Yang J., Zhu X., Hu C.T., Qiu M., Zhu Q., Ward M.D., Kahr B. Inverse correlation between lethality and thermodynamic stability of contact Insecticide polymorphs. Cryst. Growth Des. 2019;19:1839–1844. doi: 10.1021/acs.cgd.8b01800. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

