Insight into the Structure, Functions, and Dynamics of the Leptospira Outer Membrane Proteins with the Pathogenicity
- PMID: 35323775
- PMCID: PMC8951592
- DOI: 10.3390/membranes12030300
Insight into the Structure, Functions, and Dynamics of the Leptospira Outer Membrane Proteins with the Pathogenicity
Abstract
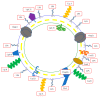
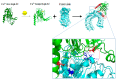
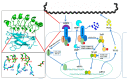
Leptospirosis is a widespread zoonosis that frequently occurs in tropical and subtropical countries. Leptospira enters the host through wounds or mucous membranes and spreads to the whole body through the blood, causing systemic infection. Kidneys are the preferential site where Leptospira accumulates, especially in the renal interstitium and renal tubule epithelial cells. Clinical symptoms in humans include high fever, jaundice, renal failure, and severe multiple-organ failure (Weil's syndrome). Surface-exposed antigens are located at the outermost layer of Leptospira and these potential virulence factors are likely involved in primary host-pathogen interactions, adhesion, and/or invasion. Using the knockout/knockdown techniques to the evaluation of pathogenicity in the virulence factor are the most direct and effective methods and many virulence factors are evaluated including lipopolysaccharides (LPS), Leptospira lipoprotein 32 (LipL32), Leptospira ompA domain protein 22 (Loa22), LipL41, LipL71, Leptospira immunoglobulin-like repeat A (LigA), LigB, and LipL21. In this review, we will discuss the structure, functions, and dynamics of these virulence factors and the roles of these virulence factors in Leptospira pathogenicity. In addition, a protein family with special Leucine-rich repeat (LRR) will also be discussed for their vital role in Leptospira pathogenicity. Finally, these surface-exposed antigens are discussed in the application of the diagnosis target for leptospirosis and compared with the serum microscope agglutination test (MAT), the gold standard for leptospirosis.
Keywords: Leptospira; Toll-like receptor; outer membrane lipoprotein; peptidoglycan.
Conflict of interest statement
The authors declare no conflict of interest and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Evaluation of LipL32 and LigA/LigB Knockdown Mutants in Leptospira interrogans Serovar Copenhageni: Impacts to Proteome and Virulence.Front Microbiol. 2022 Feb 2;12:799012. doi: 10.3389/fmicb.2021.799012. eCollection 2021. Front Microbiol. 2022. PMID: 35185824 Free PMC article.
-
LipL41 and LigA/LigB Gene Silencing on a LipL32 Knockout Leptospira interrogans Reveals the Impact of Multiple Mutations on Virulence.Pathogens. 2023 Sep 24;12(10):1191. doi: 10.3390/pathogens12101191. Pathogens. 2023. PMID: 37887707 Free PMC article.
-
Peptidoglycan mediates Leptospira outer membrane protein Loa22 to toll-like receptor 2 for inflammatory interaction: a novel innate immune recognition.Sci Rep. 2021 Jan 13;11(1):1064. doi: 10.1038/s41598-020-79662-8. Sci Rep. 2021. PMID: 33441663 Free PMC article.
-
[The Current Status of Diagnostic Tools for Leptospirosis].Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi. 2017 Aug 15;27(2):65-72. Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi. 2017. PMID: 28817942 Review. Japanese.
-
[Leptospirosis now-the centennial of the discovery of Weil's disease pathogen].Nihon Saikingaku Zasshi. 2014;69(4):589-600. doi: 10.3412/jsb.69.589. Nihon Saikingaku Zasshi. 2014. PMID: 25447984 Review. Japanese.
Cited by
-
Analysis of LruC lipoprotein and identification of peptides candidates for vaccine development and diagnosis of leptospirosis.PLoS One. 2023 Feb 6;18(2):e0281344. doi: 10.1371/journal.pone.0281344. eCollection 2023. PLoS One. 2023. PMID: 36745643 Free PMC article.
-
A Promising Tool in Serological Diagnosis: Current Research Progress of Antigenic Epitopes in Infectious Diseases.Pathogens. 2022 Sep 25;11(10):1095. doi: 10.3390/pathogens11101095. Pathogens. 2022. PMID: 36297152 Free PMC article. Review.
-
Leptospira borgpetersenii Leucine-Rich Repeat Proteins Provide Strong Protective Efficacy as Novel Leptospiral Vaccine Candidates.Trop Med Infect Dis. 2022 Dec 22;8(1):6. doi: 10.3390/tropicalmed8010006. Trop Med Infect Dis. 2022. PMID: 36668913 Free PMC article.
-
Levels of Cytokines in Leptospirosis Patients with Different Serovars and rfb Locus.J Interferon Cytokine Res. 2024 Feb;44(2):80-93. doi: 10.1089/jir.2023.0091. J Interferon Cytokine Res. 2024. PMID: 38377491 Free PMC article.
-
Cellular Pathophysiology of Leptospirosis: Role of Na/K-ATPase.Microorganisms. 2023 Jun 29;11(7):1695. doi: 10.3390/microorganisms11071695. Microorganisms. 2023. PMID: 37512868 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

