Baseline Characteristics and Outcomes After Anti-Vascular Endothelial Growth Factor Therapy for Macular Edema in Participants With Hemiretinal Vein Occlusion Compared With Participants With Central Retinal Vein Occlusion: Study of Comparative Treatments for Retinal Vein Occlusion 2 (SCORE2) Report 18
- PMID: 35323843
- PMCID: PMC8949717
- DOI: 10.1001/jamaophthalmol.2022.0352
Baseline Characteristics and Outcomes After Anti-Vascular Endothelial Growth Factor Therapy for Macular Edema in Participants With Hemiretinal Vein Occlusion Compared With Participants With Central Retinal Vein Occlusion: Study of Comparative Treatments for Retinal Vein Occlusion 2 (SCORE2) Report 18
Abstract
Importance: Intravitreal anti-vascular endothelial growth factor (VEGF) injections are commonly used to treat eyes with macular edema secondary to hemiretinal vein occlusion (HRVO) or central retinal vein occlusion (CRVO). Information on whether differences exist in outcomes after anti-VEGF therapy can help guide treatment for each of the different disease types.
Objective: To compare baseline characteristics, treatment burden, and outcomes of macular edema treatment in participants with HRVO with those of participants with CRVO.
Design, setting, and participants: This post hoc outcome analysis from the Study of Comparative Treatments for Retinal Vein Occlusion 2 randomized clinical trial included 362 participants with macular edema caused by HRVO or CRVO treated at 66 US sites. Randomization began in September 2014, and the last month 24 follow-up visit occurred in February 2018. Data were analyzed from April 2020 to May 2021.
Interventions: Eyes were initially randomized to 6 monthly intravitreal injections of aflibercept or bevacizumab and were treated according to protocol between months 6 to 12 depending on 6-month outcome. After month 12, patients were treated per investigator discretion and observed through month 60.
Main outcomes and measures: Mean visual acuity letter score (VALS).
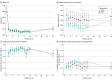
Results: Of 362 included patients, 157 (43.4%) were female, and the mean (SD) age was 68.9 (12.0) years. Outcome data were analyzed up to month 24 owing to substantial missing data at later visits. A significantly greater proportion of participants with HRVO than those with CRVO were Black (37% vs 11%). Treatment rates between months 12 to 23 were 0.36 (95% CI, 0.32-0.40) injections per month for patients with CRVO and 0.28 (95% CI, 0.19-0.36) for patients with HRVO (P = .11). The mean VALS from months 1 to 24 of an HRVO study eye exceeded that of a CRVO study eye by 5.5 (95% CI, 1.5-9.5; P = .01), consistent with the magnitude of the VALS difference between eyes with CRVO and HRVO at baseline. Eyes with CRVO presented at baseline with more macular edema than eyes with HRVO (difference in central subfield thickness [CST], 86 μm; 95% CI, 48-124; P < .001), with no difference in CST between the groups throughout months 1 to 24.
Conclusions and relevance: Black race was more prevalent among participants with HRVO than CRVO, and no differences were observed in the frequency of treatments for macular edema between eyes with CRVO and HRVO. Although eyes with CRVO presented with worse visual acuity and more macular edema on average than did eyes with HRVO, the magnitude of VALS improvement, central retinal thickness in response to anti-VEGF therapy, and treatment burden were similar between the groups.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

