Spatiotopic and retinotopic memory in the context of natural images
- PMID: 35323869
- PMCID: PMC8963666
- DOI: 10.1167/jov.22.4.11
Spatiotopic and retinotopic memory in the context of natural images
Abstract
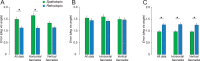
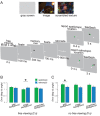
Neural responses throughout the visual cortex encode stimulus location in a retinotopic (i.e., eye-centered) reference frame, and memory for stimulus position is most precise in retinal coordinates. Yet visual perception is spatiotopic: objects are perceived as stationary, even though eye movements cause frequent displacement of their location on the retina. Previous studies found that, after a single saccade, memory of retinotopic locations is more accurate than memory of spatiotopic locations. However, it is not known whether various aspects of natural viewing affect the retinotopic reference frame advantage. We found that the retinotopic advantage may in part depend on a retinal afterimage, which can be effectively nullified through backwards masking. Moreover, in the presence of natural scenes, spatiotopic memory is more accurate than retinotopic memory, but only when subjects are provided sufficient time to process the scene before the eye movement. Our results demonstrate that retinotopic memory is not always more accurate than spatiotopic memory and that the fidelity of memory traces in both reference frames are sensitive to the presence of contextual cues.
Figures



Similar articles
-
Memory for retinotopic locations is more accurate than memory for spatiotopic locations, even for visually guided reaching.Psychon Bull Rev. 2018 Aug;25(4):1388-1398. doi: 10.3758/s13423-017-1401-x. Psychon Bull Rev. 2018. PMID: 29159799 Free PMC article.
-
Retinotopic memory is more precise than spatiotopic memory.Proc Natl Acad Sci U S A. 2012 Jan 31;109(5):1796-801. doi: 10.1073/pnas.1113168109. Epub 2012 Jan 17. Proc Natl Acad Sci U S A. 2012. PMID: 22307648 Free PMC article.
-
Attention doesn't slide: spatiotopic updating after eye movements instantiates a new, discrete attentional locus.Atten Percept Psychophys. 2011 Jan;73(1):7-14. doi: 10.3758/s13414-010-0016-3. Atten Percept Psychophys. 2011. PMID: 21258903 Free PMC article.
-
Constructing stable spatial maps of the world.Perception. 2012;41(11):1355-72. doi: 10.1068/p7392. Perception. 2012. PMID: 23513621 Review.
-
Shifts in the eye-centered frame of reference may underlie saccades, visual perception, and eye-hand coordination.J Neurophysiol. 2022 Oct 1;128(4):1025-1039. doi: 10.1152/jn.00531.2021. Epub 2022 Sep 7. J Neurophysiol. 2022. PMID: 36070246 Review.
Cited by
-
Maintaining visual stability in naturalistic scenes: The roles of trans-saccadic memory and default assumptions.Cognition. 2025 Sep;262:106165. doi: 10.1016/j.cognition.2025.106165. Epub 2025 May 10. Cognition. 2025. PMID: 40349562
-
Dynamic saccade context triggers more stable object-location binding.J Exp Psychol Gen. 2024 Apr;153(4):873-888. doi: 10.1037/xge0001545. Epub 2024 Feb 1. J Exp Psychol Gen. 2024. PMID: 38300544 Free PMC article.
-
Brain representations of motion and position in the double-drift illusion.Elife. 2024 May 29;13:e76803. doi: 10.7554/eLife.76803. Elife. 2024. PMID: 38809774 Free PMC article.
-
Navigating space and the developing mind.Front Psychol. 2025 May 14;16:1521487. doi: 10.3389/fpsyg.2025.1521487. eCollection 2025. Front Psychol. 2025. PMID: 40438758 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

