Etp1 confers arsenite resistance by affecting ACR3 expression
- PMID: 35323907
- PMCID: PMC9041338
- DOI: 10.1093/femsyr/foac018
Etp1 confers arsenite resistance by affecting ACR3 expression
Abstract
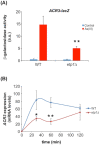
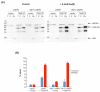
In a high-throughput yeast two-hybrid screen of predicted coiled-coil motif interactions in the Saccharomyces cerevisiae proteome, the protein Etp1 was found to interact with the yeast AP-1-like transcription factors Yap8, Yap1 and Yap6. Yap8 plays a crucial role during arsenic stress since it regulates expression of the resistance genes ACR2 and ACR3. The function of Etp1 is not well understood but the protein has been implicated in transcription and protein turnover during ethanol stress, and the etp1∆ mutant is sensitive to ethanol. In this current study, we investigated whether Etp1 is implicated in Yap8-dependent functions. We show that Etp1 is required for optimal growth in the presence of trivalent arsenite and for optimal expression of the arsenite export protein encoded by ACR3. Since Yap8 is the only known transcription factor that regulates ACR3 expression, we investigated whether Etp1 regulates Yap8. Yap8 ubiquitination, stability, nuclear localization and ACR3 promoter association were unaffected in etp1∆ cells, indicating that Etp1 affects ACR3 expression independently of Yap8. Thus, Etp1 impacts gene expression under arsenic and other stress conditions but the mechanistic details remain to be elucidated.
Keywords: Acr3; Etp1; Yap8; arsenic; metalloid.
© The Author(s) 2022. Published by Oxford University Press on behalf of FEMS.
Figures


Similar articles
-
Contribution of Yap1 towards Saccharomyces cerevisiae adaptation to arsenic-mediated oxidative stress.Biochem J. 2008 Sep 1;414(2):301-11. doi: 10.1042/BJ20071537. Biochem J. 2008. PMID: 18439143
-
Two residues in the basic region of the yeast transcription factor Yap8 are crucial for its DNA-binding specificity.PLoS One. 2013 Dec 16;8(12):e83328. doi: 10.1371/journal.pone.0083328. eCollection 2013. PLoS One. 2013. PMID: 24358276 Free PMC article.
-
Yap1 overproduction restores arsenite resistance to the ABC transporter deficient mutant ycf1 by activating ACR3 expression.Biochem Cell Biol. 2001;79(4):441-8. Biochem Cell Biol. 2001. PMID: 11527213
-
Arsenic Directly Binds to and Activates the Yeast AP-1-Like Transcription Factor Yap8.Mol Cell Biol. 2015 Dec 28;36(6):913-22. doi: 10.1128/MCB.00842-15. Mol Cell Biol. 2015. PMID: 26711267 Free PMC article.
-
Yeast AP-1 like transcription factors (Yap) and stress response: a current overview.Microb Cell. 2019 May 28;6(6):267-285. doi: 10.15698/mic2019.06.679. Microb Cell. 2019. PMID: 31172012 Free PMC article. Review.
Cited by
-
Adaptation of the yeast gene knockout collection is near-perfectly predicted by fitness and diminishing return epistasis.G3 (Bethesda). 2022 Nov 4;12(11):jkac240. doi: 10.1093/g3journal/jkac240. G3 (Bethesda). 2022. PMID: 36083011 Free PMC article.
-
Multilevel Regulation of Membrane Proteins in Response to Metal and Metalloid Stress: A Lesson from Yeast.Int J Mol Sci. 2024 Apr 18;25(8):4450. doi: 10.3390/ijms25084450. Int J Mol Sci. 2024. PMID: 38674035 Free PMC article. Review.
References
-
- Ahmadpour D, Maciaszczyk-Dziubinska E, Babazadeh Ret al. . The mitogen-activated protein kinase Slt2 modulates arsenite transport through the aquaglyceroporin Fps1. FEBS Lett. 2016;590:3649–59. - PubMed
-
- Bobrowicz P, Wysocki R, Owsianik Get al. . Isolation of three contiguous genes, ACR1, ACR2 and ACR3, involved in resistance to arsenic compounds in the yeast Saccharomycescerevisiae. Yeast. 1997;13:819–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

