CCK and GLP-1 release in response to proteinogenic amino acids using a small intestine ex vivo model in pigs
- PMID: 35323927
- PMCID: PMC9030139
- DOI: 10.1093/jas/skac093
CCK and GLP-1 release in response to proteinogenic amino acids using a small intestine ex vivo model in pigs
Abstract
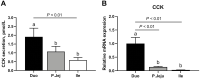
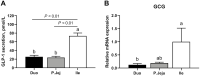
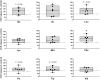
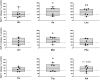
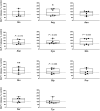
The impact of individual amino acids (AA) on gut hormone secretion and appetite regulation in pigs remains largely unknown. The aim of the present study was to determine the effect of the 20 proteinogenic AA on the release of the anorexigenic hormones cholecystokinin (CCK) and glucagon-like peptide 1 (GLP-1) in postweaning pigs. Six 25-d-old male piglets (Domestic Landrace × Large White; body weight = 6.94 ± 0.29 kg) were humanely killed for the collection of intestinal segments from the duodenum, jejunum, and ileum. Tissue samples from the three intestinal segments were used to determine which of the regions were more relevant for the analysis of gut peptides. Only the segments with the highest CCK and GLP-1 secretion and expression levels were evaluated with the 20 individual AA. Tissue segments were cut open, cleaned, and stripped of their muscle layer before identical circular samples were collected and incubated in 24-well plates for 1 h (37 °C, 5% v/v CO2). The culture broth consisted of a glucose-free KRB buffer containing no added AA (control) or with the addition of 10 mM of 1 of the 20 proteinogenic AA. Following incubation, tissues and supernatant were collected for gene expression and secretion analysis of CCK and GLP-1 levels. CCK secretion and mRNA expression were higher (P < 0.05) in duodenum when compared with proximal jejunum or ileum, whereas GLP-1/proglucagon levels were higher in ileum vs. duodenum (P < 0.05) and jejunum (P < 0.05, for GLP-1 only) in postweaning pigs. Based on these results, the effect of AA on CCK and GLP-1 secretion was studied in the duodenum and ileum, respectively. None of the AA tested stimulated both anorexigenic hormones. Of all the essential AA, Ile, Leu, Met, and Trp significantly (P < 0.05) stimulated GLP-1 from the ileum, while only Phe stimulated CCK from the duodenum. Of the nonessential AA, amide AA (Gln and Asn) caused the release of CCK, while Glu and Arg increased the release of GLP-1 from the ileum. Interpreting the results in the context of the digestion and absorption dynamics, non-bound AA are quickly absorbed and have their effect on gut peptide secretion limited to the proximal small intestine (i.e., duodenum), thus, mainly CCK. In contrast, protein-bound AA would only stimulate CCK release from the duodenum through feedback mechanisms (such as through GLP-1 secreted mainly in the ileum).
Keywords: amino acid; cholecystokinin; gastrointestinal tract; glucagon-like peptide 1; pig.
Plain language summary
Understanding which dietary amino acids (AA) may impact the release of gut hormones involved in the modulation of feed intake, such as cholecystokinin (CCK) and glucagon-like peptide 1 (GLP-1), can help improve pig feed formulations. The series of studies presented assessed the effect of the 20 proteinogenic non-bound AA on the secretion of CCK and/or GLP-1 by duodenum, jejunum, and/or ileum samples from postweaning piglets. None of the AA tested stimulated the secretion of both CCK and GLP-1. Among the essential AA (EAA), Ile, Leu, Met, and Trp significantly stimulated GLP-1 from the ileum, while Phe stimulated CCK from the duodenum. Of the non-essential AA (NEAA), AA amides Gln and Asn caused the release of CCK, while Glu and Arg increased the release of GLP-1 from the ileum. The results suggest that both non-bound EAA and NEAA participate in appetite control via the release of gut peptides in pigs. Given that CCK was mainly released from duodenum samples (in the pre-enzymatic section of the small intestine), protein-bound AA could only influence CCK release through feedback mechanisms such as through the presence of GLP-1 receptors.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Leucine (and lysine) increased plasma levels of the satiety hormone cholecystokinin (CCK), and phenylalanine of the incretin glucagon-like peptide 1 (GLP-1) after oral gavages in pigs.J Anim Sci. 2023 Jan 3;101:skad175. doi: 10.1093/jas/skad175. J Anim Sci. 2023. PMID: 37233611 Free PMC article.
-
CCK-1 and CCK-2 receptor agonism do not stimulate GLP-1 and neurotensin secretion in the isolated perfused rat small intestine or GLP-1 and PYY secretion in the rat colon.Physiol Rep. 2020 Jan;8(2):e14352. doi: 10.14814/phy2.14352. Physiol Rep. 2020. PMID: 31984675 Free PMC article.
-
Soybean protein hydrolysate stimulated cholecystokinin secretion and inhibited feed intake through calcium-sensing receptors and intracellular calcium signalling in pigs.Food Funct. 2021 Oct 4;12(19):9286-9299. doi: 10.1039/d1fo01596f. Food Funct. 2021. PMID: 34606544
-
Critical review evaluating the pig as a model for human nutritional physiology.Nutr Res Rev. 2016 Jun;29(1):60-90. doi: 10.1017/S0954422416000020. Epub 2016 May 13. Nutr Res Rev. 2016. PMID: 27176552 Review.
-
Review: Chemosensing of nutrients and non-nutrients in the human and porcine gastrointestinal tract.Animal. 2019 Nov;13(11):2714-2726. doi: 10.1017/S1751731119001794. Epub 2019 Aug 7. Animal. 2019. PMID: 31387651 Review.
Cited by
-
Impact of fermented bamboo powder on the morphology and physiology of the gastrointestinal tract in yellow-feather broiler chickens.Poult Sci. 2025 Feb;104(2):104793. doi: 10.1016/j.psj.2025.104793. Epub 2025 Jan 14. Poult Sci. 2025. PMID: 39813869 Free PMC article.
-
A Lysozyme-Derived Peptide Induces GLP-1 Secretion in Mouse Jejunal Organoids and Modulates Glycemia in Rats.J Agric Food Chem. 2025 Jul 2;73(26):16420-16428. doi: 10.1021/acs.jafc.5c05148. Epub 2025 Jun 18. J Agric Food Chem. 2025. PMID: 40528814 Free PMC article.
-
Leucine (and lysine) increased plasma levels of the satiety hormone cholecystokinin (CCK), and phenylalanine of the incretin glucagon-like peptide 1 (GLP-1) after oral gavages in pigs.J Anim Sci. 2023 Jan 3;101:skad175. doi: 10.1093/jas/skad175. J Anim Sci. 2023. PMID: 37233611 Free PMC article.
-
An oral gavage of lysine elicited early satiation while gavages of lysine, leucine, or isoleucine prolonged satiety in pigs.J Anim Sci. 2022 Dec 1;100(12):skac361. doi: 10.1093/jas/skac361. J Anim Sci. 2022. PMID: 36315475 Free PMC article.
-
Influence of Nano-Methionine supplementation in drinking water on growth performance, lipid metabolism, and related gene expression in broiler chicken.J Adv Vet Anim Res. 2022 Dec 31;9(4):743-753. doi: 10.5455/javar.2022.i644. eCollection 2022 Dec. J Adv Vet Anim Res. 2022. PMID: 36714509 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

