Nontransgenic Guinea Pig Strains Exhibit Hallmarks of Human Brain Aging and Alzheimer's Disease
- PMID: 35323931
- PMCID: PMC9434446
- DOI: 10.1093/gerona/glac073
Nontransgenic Guinea Pig Strains Exhibit Hallmarks of Human Brain Aging and Alzheimer's Disease
Abstract
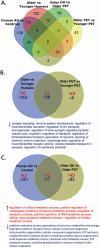
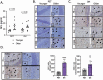
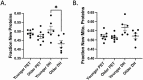
Older age is the primary risk factor for most chronic diseases, including Alzheimer's disease (AD). Current preclinical models to study brain aging and AD are mainly transgenic and harbor mutations intended to mirror brain pathologies associated with human brain aging/AD (eg, by increasing production of the amyloid precursor protein, amyloid beta [Aβ], and/or phosphorylated tau, all of which are key pathological mediators of AD). Although these models may provide insight on pathophysiological processes in AD, none completely recapitulate the disease and its strong age-dependence, and there has been limited success in translating preclinical results and treatments to humans. Here, we describe 2 nontransgenic guinea pig (GP) models, a standard PigmEnTed (PET) strain, and lesser-studied Dunkin-Hartley (DH) strain, that may naturally mimic key features of brain aging and AD in humans. We show that brain aging in PET GP is transcriptomically similar to human brain aging, whereas older DH brains are transcriptomically more similar to human AD. Both strains/models also exhibit increased neurofilament light chain (NFL, a marker of neuronal damage) with aging, and DH animals display greater S100 calcium-binding protein B (S100β), ionized calcium-binding adapter molecule 1 (Iba1), and Aβ and phosphorylated tau-which are all important markers of neuroinflammation-associated AD. Collectively, our results suggest that both the PET and DH GP may be useful, nontransgenic models to study brain aging and AD, respectively.
Keywords: Alzheimer’s disease; Brain aging; Inflammation; Transcriptomics.
© The Author(s) 2022. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

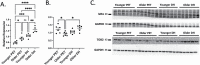
Similar articles
-
Aging, cortical injury and Alzheimer's disease-like pathology in the guinea pig brain.Neurobiol Aging. 2014 Jun;35(6):1345-51. doi: 10.1016/j.neurobiolaging.2013.11.020. Epub 2013 Nov 27. Neurobiol Aging. 2014. PMID: 24360504
-
Soluble Conformers of Aβ and Tau Alter Selective Proteins Governing Axonal Transport.J Neurosci. 2016 Sep 14;36(37):9647-58. doi: 10.1523/JNEUROSCI.1899-16.2016. J Neurosci. 2016. PMID: 27629715 Free PMC article.
-
Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer's disease.Hum Mol Genet. 2012 Dec 1;21(23):5131-46. doi: 10.1093/hmg/dds360. Epub 2012 Aug 27. Hum Mol Genet. 2012. PMID: 22926141 Free PMC article.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
Cited by
-
Alzheimer's Disease Neuropathological Change in Aged Non-Primate Mammals.Int J Mol Sci. 2024 Jul 25;25(15):8118. doi: 10.3390/ijms25158118. Int J Mol Sci. 2024. PMID: 39125687 Free PMC article. Review.
-
Nanoligomers targeting NF-κB and NLRP3 reduce neuroinflammation and improve cognitive function with aging and tauopathy.J Neuroinflammation. 2024 Jul 27;21(1):182. doi: 10.1186/s12974-024-03182-9. J Neuroinflammation. 2024. PMID: 39068433 Free PMC article.
-
Protective effects of apigenin on the brain transcriptome with aging.Mech Ageing Dev. 2024 Feb;217:111889. doi: 10.1016/j.mad.2023.111889. Epub 2023 Nov 24. Mech Ageing Dev. 2024. PMID: 38007051 Free PMC article.
-
Non-transgenic guinea pig strains exhibit divergent age-related changes in hippocampal mitochondrial respiration.Acta Physiol (Oxf). 2024 Aug;240(8):e14185. doi: 10.1111/apha.14185. Epub 2024 Jun 11. Acta Physiol (Oxf). 2024. PMID: 38860650 Free PMC article.
-
Immune cell infiltration and modulation of the blood-brain barrier in a guinea pig model of tuberculosis: Observations without evidence of bacterial dissemination to the brain.PLoS One. 2024 Dec 31;19(12):e0307577. doi: 10.1371/journal.pone.0307577. eCollection 2024. PLoS One. 2024. PMID: 39739680 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

