Facilitating Safe Discharge Through Predicting Disease Progression in Moderate Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study to Develop and Validate a Clinical Prediction Model in Resource-Limited Settings
- PMID: 35323932
- PMCID: PMC9129107
- DOI: 10.1093/cid/ciac224
Facilitating Safe Discharge Through Predicting Disease Progression in Moderate Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study to Develop and Validate a Clinical Prediction Model in Resource-Limited Settings
Abstract
Background: In locations where few people have received coronavirus disease 2019 (COVID-19) vaccines, health systems remain vulnerable to surges in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. Tools to identify patients suitable for community-based management are urgently needed.
Methods: We prospectively recruited adults presenting to 2 hospitals in India with moderate symptoms of laboratory-confirmed COVID-19 to develop and validate a clinical prediction model to rule out progression to supplemental oxygen requirement. The primary outcome was defined as any of the following: SpO2 < 94%; respiratory rate > 30 BPM; SpO2/FiO2 < 400; or death. We specified a priori that each model would contain three clinical parameters (age, sex, and SpO2) and 1 of 7 shortlisted biochemical biomarkers measurable using commercially available rapid tests (C-reactive protein [CRP], D-dimer, interleukin 6 [IL-6], neutrophil-to-lymphocyte ratio [NLR], procalcitonin [PCT], soluble triggering receptor expressed on myeloid cell-1 [sTREM-1], or soluble urokinase plasminogen activator receptor [suPAR]), to ensure the models would be suitable for resource-limited settings. We evaluated discrimination, calibration, and clinical utility of the models in a held-out temporal external validation cohort.
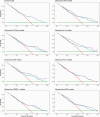
Results: In total, 426 participants were recruited, of whom 89 (21.0%) met the primary outcome; 257 participants comprised the development cohort, and 166 comprised the validation cohort. The 3 models containing NLR, suPAR, or IL-6 demonstrated promising discrimination (c-statistics: 0.72-0.74) and calibration (calibration slopes: 1.01-1.05) in the validation cohort and provided greater utility than a model containing the clinical parameters alone.
Conclusions: We present 3 clinical prediction models that could help clinicians identify patients with moderate COVID-19 suitable for community-based management. The models are readily implementable and of particular relevance for locations with limited resources.
Trial registration: ClinicalTrials.gov NCT04441372.
Keywords: COVID-19; LMIC; low- and middle-income country; prognostic model; triage.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


References
-
- Ritchie H, Mathieu E, Rodes-Guirao L, et al. . Coronavirus pandemic (COVID-19)—vaccinations. 2020. Available at: https://ourworldindata.org/covid-vaccinations. Accessed 21 December 2021.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

