Elongin C (ELOC/TCEB1)-associated von Hippel-Lindau disease
- PMID: 35323939
- PMCID: PMC9402235
- DOI: 10.1093/hmg/ddac066
Elongin C (ELOC/TCEB1)-associated von Hippel-Lindau disease
Abstract
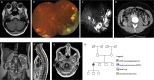
Around 95% of patients with clinical features that meet the diagnostic criteria for von Hippel-Lindau disease (VHL) have a detectable inactivating germline variant in VHL. The VHL protein (pVHL) functions as part of the E3 ubiquitin ligase complex comprising pVHL, elongin C, elongin B, cullin 2 and ring box 1 (VCB-CR complex), which plays a key role in oxygen sensing and degradation of hypoxia-inducible factors. To date, only variants in VHL have been shown to cause VHL disease. We undertook trio analysis by whole-exome sequencing in a proband with VHL disease but without a detectable VHL mutation. Molecular studies were also performed on paired DNA extracted from the proband's kidney tumour and blood and bioinformatics analysis of sporadic renal cell carcinoma (RCC) dataset was undertaken. A de novo pathogenic variant in ELOC NM_005648.4(ELOC):c.236A>G (p.Tyr79Cys) gene was identified in the proband. ELOC encodes elongin C, a key component [C] of the VCB-CR complex. The p.Tyr79Cys substitution is a mutational hotspot in sporadic VHL-competent RCC and has previously been shown to mimic the effects of pVHL deficiency on hypoxic signalling. Analysis of an RCC from the proband showed similar findings to that in somatically ELOC-mutated RCC (expression of hypoxia-responsive proteins, no somatic VHL variants and chromosome 8 loss). These findings are consistent with pathogenic ELOC variants being a novel cause for VHL disease and suggest that genetic testing for ELOC variants should be performed in individuals with suspected VHL disease with no detectable VHL variant.
© The Author(s) 2022. Published by Oxford University Press.
Figures



References
-
- Gossage, L., Eisen, T. and Maher, E.R. (2015) VHL, the story of a tumour suppressor gene. Nat. Rev. Cancer, 15, 55–64. - PubMed
-
- Maher, E.R., Yates, J.R., Harries, R., Benjamin, C., Harris, R., Moore, A.T. and Ferguson-Smith, M.A. (1990) Clinical features and natural history of von Hippel-Lindau disease. Q. J. Med., 77, 1151–1163. - PubMed
-
- Melmon, K.L. and Rosen, S.W. (1964) Lindau’s disease. Review of the literature and study of a large kindred. Am. J. Med., 36, 595–617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

