Early phenotypic detection of fluconazole- and anidulafungin-resistant Candida glabrata isolates
- PMID: 35323941
- PMCID: PMC9840476
- DOI: 10.1093/jac/dkac075
Early phenotypic detection of fluconazole- and anidulafungin-resistant Candida glabrata isolates
Abstract
Background: Increased fluconazole and echinocandin resistance in Candida glabrata requires prompt detection in routine settings. A phenotypic test based on the EUCAST E.DEF 7.3.2 protocol was developed for the detection of fluconazole- and anidulafungin-resistant isolates utilizing the colorimetric dye XTT.
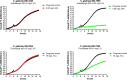
Methods: Thirty-one clinical C. glabrata isolates, 11 anidulafungin resistant and 14 fluconazole resistant, were tested. After optimization studies, 0.5-2.5 × 105 cfu/mL of each isolate in RPMI 1640 + 2% d-glucose medium containing 100 mg/L XTT + 0.78 μΜ menadione and 0.06 mg/L anidulafungin (S breakpoint) or 16 mg/L fluconazole (I breakpoint) in 96-well flat-bottom microtitration plates were incubated at 37°C for 18 h; we also included drug-free wells. XTT absorbance was measured at 450 nm every 15 min. Differences between the drug-free and the drug-treated wells were assessed using Student's t-test at different timepoints. ROC curves were used in order to identify the best timepoint and cut-off.
Results: The XTT absorbance differences between fluconazole-containing and drug-free wells were significantly lower for the resistant isolates compared with susceptible increased exposure isolates (0.08 ± 0.05 versus 0.25 ± 0.06, respectively, P = 0.005) at 7.5 h, with a difference of <0.157 corresponding to 100% sensitivity and 94% specificity for detection of resistance. The XTT absorbance differences between anidulafungin-containing and drug-free wells were significantly lower for the resistant isolates compared with susceptible isolates (0.08 ± 0.07 versus 0.200 ± 0.03, respectively, P < 0.001) at 5 h, with a difference of <0.145 corresponding to 91% sensitivity and 100% specificity, irrespective of underlying mutations.
Conclusions: A simple, cheap and fast phenotypic test was developed for detection of fluconazole- and anidulafungin-resistant C. glabrata isolates.
© The Author(s) 2022. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Detecting Echinocandin Resistance in C. glabrata Using Commercial Methods: Are CLSI or EUCAST Breakpoints Suitable for Categorical Classification?Mycoses. 2024 Nov;67(11):e70003. doi: 10.1111/myc.70003. Mycoses. 2024. PMID: 39567221
-
Detection of Echinocandin-Resistant Candida glabrata in Blood Cultures Spiked with Different Percentages of FKS2 Mutants.Antimicrob Agents Chemother. 2019 Feb 26;63(3):e02004-18. doi: 10.1128/AAC.02004-18. Print 2019 Mar. Antimicrob Agents Chemother. 2019. PMID: 30559139 Free PMC article.
-
Mutant Prevention Concentration and Mutant Selection Window of Micafungin and Anidulafungin in Clinical Candida glabrata Isolates.Antimicrob Agents Chemother. 2018 Feb 23;62(3):e01982-17. doi: 10.1128/AAC.01982-17. Print 2018 Mar. Antimicrob Agents Chemother. 2018. PMID: 29311063 Free PMC article.
-
Resistance in human pathogenic yeasts and filamentous fungi: prevalence, underlying molecular mechanisms and link to the use of antifungals in humans and the environment.Dan Med J. 2016 Oct;63(10):B5288. Dan Med J. 2016. PMID: 27697142 Review.
Cited by
-
Molecular investigations on Candida glabrata clinical isolates for pharmacological targeting.RSC Adv. 2022 Jun 14;12(27):17570-17584. doi: 10.1039/d2ra02092k. eCollection 2022 Jun 7. RSC Adv. 2022. PMID: 35765448 Free PMC article.
-
Prevalence and molecular basis of resistance to echinocandins among clinical Candida glabrata isolates in Guangdong province, China.BMC Microbiol. 2025 Aug 20;25(1):521. doi: 10.1186/s12866-025-04275-y. BMC Microbiol. 2025. PMID: 40830434 Free PMC article.
-
Preclinical Models for Cryptococcosis of the CNS and Their Characterization Using In Vivo Imaging Techniques.J Fungi (Basel). 2024 Feb 12;10(2):146. doi: 10.3390/jof10020146. J Fungi (Basel). 2024. PMID: 38392818 Free PMC article. Review.
References
-
- Pappas PG, Lionakis MS, Arendrup MCet al. . Invasive candidiasis. Nat Rev Dis Primers 2018; 4: 18026. - PubMed
-
- Sanguinetti M, Posteraro B, Lass-Flörl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses 2015; 58: 2–13. - PubMed
-
- Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect 2019; 25: 792–8. - PubMed
-
- Arendrup MC, Patterson TF. Multidrug-resistant Candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis 2017; 216Suppl 3: S445–51. - PubMed

