DNA Tension Probes Show that Cardiomyocyte Maturation Is Sensitive to the Piconewton Traction Forces Transmitted by Integrins
- PMID: 35324164
- PMCID: PMC11238821
- DOI: 10.1021/acsnano.1c04303
DNA Tension Probes Show that Cardiomyocyte Maturation Is Sensitive to the Piconewton Traction Forces Transmitted by Integrins
Abstract
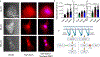
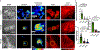
Cardiac muscle cells (CMCs) are the unit cells that comprise the heart. CMCs go through different stages of differentiation and maturation pathways to fully mature into beating cells. These cells can sense and respond to mechanical cues through receptors such as integrins which influence maturation pathways. For example, cell traction forces are important for the differentiation and development of functional CMCs, as CMCs cultured on varying substrate stiffness function differently. Most work in this area has focused on understanding the role of bulk extracellular matrix stiffness in mediating the functional fate of CMCs. Given that stiffness sensing mechanisms are mediated by individual integrin receptors, an important question in this area pertains to the specific magnitude of integrin piconewton (pN) forces that can trigger CMC functional maturation. To address this knowledge gap, we used DNA adhesion tethers that rupture at specific thresholds of force (∼12, ∼56, and ∼160 pN) to test whether capping peak integrin tension to specific magnitudes affects CMC function. We show that adhesion tethers with greater force tolerance lead to functionally mature CMCs as determined by morphology, twitching frequency, transient calcium flux measurements, and protein expression (F-actin, vinculin, α-actinin, YAP, and SERCA2a). Additionally, sarcomeric actinin alignment and multinucleation were significantly enhanced as the mechanical tolerance of integrin tethers was increased. Taken together, the results show that CMCs harness defined pN integrin forces to influence early stage development. This study represents an important step toward biophysical characterization of the contribution of pN forces in early stage cardiac differentiation.
Keywords: DNA sensors; cardiomyocyte’s maturation; integrin forces; integrin mechanotransduction; pN forces; rupture probes; substrate stiffness.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

