Highly Selective Synthesis of Seven-Membered Azaspiro Compounds by a Rh(I)-Catalyzed Cycloisomerization/Diels-Alder Cascade of 1,5-Bisallenes
- PMID: 35324177
- PMCID: PMC9016767
- DOI: 10.1021/acs.joc.2c00065
Highly Selective Synthesis of Seven-Membered Azaspiro Compounds by a Rh(I)-Catalyzed Cycloisomerization/Diels-Alder Cascade of 1,5-Bisallenes
Abstract
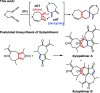
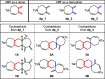
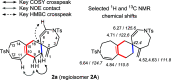
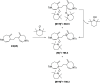
The synthesis of spiro compounds featuring seven- and six-membered rings in the spirobicyclic motif is successfully achieved through a cascade process encompassing a rhodium(I)-catalyzed cycloisomerization followed by a highly selective Diels-Alder homodimerization. The scope of the reaction is analyzed based on a series of synthetic substrates, and control experiments and DFT calculations led us to justify the exquisite degree of selectivity observed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Pradhan R.; Patra M.; Behera A. K.; Mishra B. K.; Behera R. K. A synthon approach to spiro compounds. Tetrahedron 2006, 62, 779–828. 10.1016/j.tet.2005.09.039. - DOI
-
-
For a review, see:
- Abdul R.; Xufeng L. Development and application of chiral spirocyclic phosphoric acids in asymmetric catalysis. Org. Biomol. Chem. 2018, 16, 4753–4777. 10.1039/C8OB00900G. - DOI - PubMed
-
For selected examples, see:
- Xie J.-H.; Duan H.-F.; Fan B.-M.; Cheng X.; Wang L.-X.; Zhou Q.-L. Application of SDP ligands for Pd-catalyzed allylic alkylation. Adv. Synth. Catal. 2004, 346, 625–632. 10.1002/adsc.200404003. - DOI
- Xu C.; Qi Y.; Yang X.; Li X.; Li Z.; Bai L. Development of C2-symmetric chiral spirocyclic phase-transfer catalysts: synthesis and application to asymmetric alkylation of glycinate Schiff base. Org. Lett. 2021, 23, 2890–2894. 10.1021/acs.orglett.1c00535. - DOI - PubMed
- Cheng X.; Zhang Q.; Xie J.-H.; Wang L.-X.; Zhou Q.-L. Highly rigid diphosphane ligands with a large dihedral angle based on a chiral spirobifluorene backbone. Angew. Chem., Int. Ed. 2005, 44, 1118–1121. 10.1002/anie.200462072. - DOI - PubMed
-
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

