Biosimilar Versus Originator Pegfilgrastim for Preventing Chemotherapy-Induced Neutropenia: A Phase III Randomized, Multicenter, Evaluator-Blinded, Noninferiority Study
- PMID: 35324270
- PMCID: PMC9071253
- DOI: 10.1200/GO.21.00276
Biosimilar Versus Originator Pegfilgrastim for Preventing Chemotherapy-Induced Neutropenia: A Phase III Randomized, Multicenter, Evaluator-Blinded, Noninferiority Study
Abstract
Purpose: This study evaluated the efficacy, safety, and immunogenicity of biosimilar pegfilgrastim (PegFilBS) and originator pegfilgrastim (PegFilOR) in patients with stage 2-4 breast cancer.
Methods: This phase III randomized, multicenter, evaluator-blinded, noninferiority study recruited women with stage 2-4 breast cancer in Argentina who were scheduled to receive chemotherapy. Stratification was based on the breast cancer stage. The primary end point was the duration of severe neutropenia (DSN, noninferiority margin: 1 day) in the first chemotherapy cycle. Secondary end points assessed were incidence of severe neutropenia, grade 3 neutropenia, febrile neutropenia, infections, postchemotherapy hospitalization and duration, and the incidence of adverse drug reactions (ADRs).
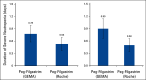
Results: A total of 120 patients were randomly assigned to receive PegFilBS (58 patients) or PegFilOR (62 patients). Severe neutropenia occurred in 52 of 283 cycles (18.4%) for 27 patients who received PegFilBS and in 48 of 297 cycles (16.2%) for 20 patients who received PegFilOR (P = .48). During the first cycle, severe neutropenia occurred in 16 patients who received PegFilBS (DSN: 0.78 ± 1.53 days) and in 11 patients who received PegFilOR (DSN: 0.53 ± 1.25 days; 95% CI, -0.26 to 0.76 days). In the intention-to-treat analysis, the mean DSN values were 0.90 ± 1.79 days for the PegFilBS group and 0.50 ± 1.21 for the PegFilOR group (95% CI, -0.15 to 0.95 days). No significant differences were observed for the secondary efficacy end points. Three patients experienced seven ADRs in the PegFilBS group while 10 patients experienced 31 ADRs in the PegFilOR group. The most common ADR was myalgia.
Conclusion: Relative to PegFilOR, PegFilBS provided noninferior efficacy outcomes in Argentinian women with stage 2-4 breast cancer who were treated using myelosuppressive chemotherapy.
Conflict of interest statement
Figures
References
-
- Kuderer NM, Dale DC, Crawford J, et al. : Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258-2266, 2006 - PubMed
-
- Committee for Medicinal Products for Human Use (CHMP) : Annex to Guideline on Similar Biological Medicinal Products Containing Biotechnology-Derived Proteins as Active Substance: Non-Clinical and Clinical Issues. Guidance on Similar Medicinal Products Containing Recombinant Granulocyte-Colony Stimulating Factor. EMEA/CHMP/BMWP/31329/2005. London UK, 2006
-
- Souza LM‚ Boone TC‚ Gabrilove J‚ et al. : Recombinant human granulocyte colonystimulating factor: Effects on normal and leukemic myeloid cells. Science 232:61-65, 1986 - PubMed
-
- Weisbart RH‚ Kacena A‚ Schuh A‚ et al. : GM-CSF induces human neutrophil IgA-mediated phagocytosis by an IgA Fc receptor activation mechanism. Nature 332:647-648, 1988 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials