New Insights into the Toxin Diversity and Antimicrobial Activity of the "Fire Coral" Millepora complanata
- PMID: 35324703
- PMCID: PMC8954376
- DOI: 10.3390/toxins14030206
New Insights into the Toxin Diversity and Antimicrobial Activity of the "Fire Coral" Millepora complanata
Abstract
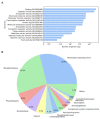
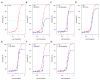
To date, few studies have been carried out aimed at characterizing the toxins synthesized by hydrocorals of the genus Millepora. The purpose of this study was to explore the toxin diversity and antibacterial activity of the "fire coral" M. complanata using a transcriptomic data mining approach. In addition, the cytolytic and antibacterial activities of the M. complanata nematocyst proteome were experimentally confirmed. Cytolysins were predicted from the transcriptome by comparing against the Animal Toxin Annotation Project database, resulting in 190 putative toxins, including metalloproteases, hemostasis-impairing toxins, phospholipases, among others. The M. complanata nematocyst proteome was analyzed by 1D and 2D electrophoresis and zymography. The zymograms showed different zones of cytolytic activity: two zones of hemolysis at ~25 and ~205 kDa, two regions corresponding to phospholipase A2 (PLA2) activity around 6 and 25 kDa, and a proteolytic zone was observed between 50 and 205 kDa. The hemolytic activity of the proteome was inhibited in the presence of PLA2 and proteases inhibitors, suggesting that PLA2s, trypsin, chymotrypsin, serine-proteases, and matrix metalloproteases are responsible for the hemolysis. On the other hand, antimicrobial peptide sequences were retrieved from their transcripts with the amPEPpy software. This analysis revealed the presence of homologs to SK84, cgUbiquitin, Ubiquicidin, TroTbeta4, SPINK9-v1, and Histone-related antimicrobials in the transcriptome of this cnidarian. Finally, by employing disk diffusion and microdilution assays, we found that the nematocyst peptidome of M. complanata showed inhibitory activity against both Gram-positive and Gram-negative bacteria including S. enteritidis, P. perfectomarina, E. coli, and C. xerosis, among others. This is the first transcriptomic data mining analysis to explore the diversity of the toxins synthesized by an organism of the genus Millepora. Undoubtedly, this work provides information that will broaden our general understanding of the structural richness of cnidarian toxins.
Keywords: Millepora complanata; antibacterial peptides; cytolysins; toxin diversity; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Transcriptomic and proteomic analyses reveal the first occurrence of diverse toxin groups in Millepora alcicornis.J Proteomics. 2023 Sep 30;288:104984. doi: 10.1016/j.jprot.2023.104984. Epub 2023 Aug 1. J Proteomics. 2023. PMID: 37536522
-
Comparative Analysis of the Soluble Proteome and the Cytolytic Activity of Unbleached and Bleached Millepora complanata ("Fire Coral") from the Mexican Caribbean.Mar Drugs. 2019 Jul 3;17(7):393. doi: 10.3390/md17070393. Mar Drugs. 2019. PMID: 31277227 Free PMC article.
-
Biochemical and pharmacological characterization of toxins obtained from the fire coral Millepora complanata.Comp Biochem Physiol C Toxicol Pharmacol. 2007 Nov;146(4):511-8. doi: 10.1016/j.cbpc.2007.06.002. Epub 2007 Jun 14. Comp Biochem Physiol C Toxicol Pharmacol. 2007. PMID: 17644443
-
Millepora species (Fire Coral) Sting: A Case Report and Review of Recommended Management.Wilderness Environ Med. 2018 Dec;29(4):521-526. doi: 10.1016/j.wem.2018.06.012. Epub 2018 Sep 17. Wilderness Environ Med. 2018. PMID: 30236886 Review.
-
[Cytolysins and homologous proteins produced by gram-negative bacteria. I. Hemolysis and leukotoxins of Escherichia coli, Bordetella pertussis, Pasteurella haemolytica and Actinobacillus sp].Postepy Hig Med Dosw. 1996;50(2):173-90. Postepy Hig Med Dosw. 1996. PMID: 8848425 Review. Polish.
Cited by
-
A novel polypeptide from stony coral Goniopora columna inhibits thrombosis by interfering with the contact-kinin pathway.Arch Toxicol. 2025 Jun 4. doi: 10.1007/s00204-025-04090-4. Online ahead of print. Arch Toxicol. 2025. PMID: 40464975
-
Unlocking Antimicrobial Peptides: In Silico Proteolysis and Artificial Intelligence-Driven Discovery from Cnidarian Omics.Molecules. 2025 Jan 25;30(3):550. doi: 10.3390/molecules30030550. Molecules. 2025. PMID: 39942653 Free PMC article.
-
Zinc speciation promotes distinct effects on dinoflagellate growth and coral trypsin-like enzyme activity.Biometals. 2025 Apr;38(2):573-586. doi: 10.1007/s10534-025-00664-y. Epub 2025 Jan 15. Biometals. 2025. PMID: 39810029
-
Assessing the Utility of Broad-Acting Inhibitors as Therapeutics in Diverse Venoms.Toxins (Basel). 2025 Apr 8;17(4):188. doi: 10.3390/toxins17040188. Toxins (Basel). 2025. PMID: 40278686 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

