Transient Post-Natal Exposure to Xenoestrogens Induces Long-Term Alterations in Cardiac Calcium Signaling
- PMID: 35324727
- PMCID: PMC8954167
- DOI: 10.3390/toxics10030102
Transient Post-Natal Exposure to Xenoestrogens Induces Long-Term Alterations in Cardiac Calcium Signaling
Abstract
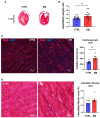
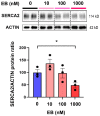
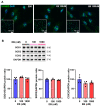
Today, non-communicable disorders are widespread worldwide. Among them, cardiovascular diseases represent the main cause of death. At the origin of these diseases, exposure to challenges during developmental windows of vulnerability (peri-conception, in utero, and early infancy periods) have been incriminated. Among the challenges that have been described, endocrine disruptors are of high concern because of their omnipresence in the environment. Worrisomely, since birth, children are exposed to a significant number of endocrine disruptors. However, the role of such early exposure on long-term cardiac health is poorly described. In this context, based on a model of rats exposed postnatally and transiently to an estrogenic compound prototype (estradiol benzoate, EB), we aimed to delineate the effects on the adult heart of such transient early exposure to endocrine disruptors and identify the underlying mechanisms involved in the potential pathogenesis. We found that this transient post-natal exposure to EB induced cardiac hypertrophy in adulthood, with increased cardiomyocyte size. The evaluation of cardiac calcium signaling, through immunoblot approaches, highlighted decreased expression of the sarcoplasmic reticulum calcium ATPase 2 (SERCA2) and decreased Nuclear Factor of Activated T Cells (NFAT3) phosphorylation as a potential underlying mechanism of cardiac hypertrophy. Furthermore, the treatment of cardiomyocytes with EB in vitro induced a decrease in SERCA2 protein levels. Overall, our study demonstrates that early transient exposure to EB induces permanent cardiac alterations. Together, our data highlight SERCA2 down-regulation as a potential mechanism involved in the cardiac pathogenesis induced by EB. These results suggest programming of adult heart dysfunctions such as arrhythmia and heart failures by early exposure to endocrine disruptors and could open new perspectives for treatment and prevention.
Keywords: calcium signaling; endocrine disruptor; heart; post-natal; programming.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Charles M.A., Delpierre C., Breant B. Developmental origin of health and adult diseases (DOHaD): Evolution of a concept over three decades. Med. Sci. 2016;32:15–20. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

