Cell Culture Process Scale-Up Challenges for Commercial-Scale Manufacturing of Allogeneic Pluripotent Stem Cell Products
- PMID: 35324781
- PMCID: PMC8945133
- DOI: 10.3390/bioengineering9030092
Cell Culture Process Scale-Up Challenges for Commercial-Scale Manufacturing of Allogeneic Pluripotent Stem Cell Products
Abstract
Allogeneic cell therapy products, such as therapeutic cells derived from pluripotent stem cells (PSCs), have amazing potential to treat a wide variety of diseases and vast numbers of patients globally. However, there are various challenges related to manufacturing PSCs in single-use bioreactors, particularly at larger volumetric scales. This manuscript addresses these challenges and presents potential solutions to alleviate the anticipated bottlenecks for commercial-scale manufacturing of high-quality therapeutic cells derived from PSCs.
Keywords: allogeneic cell therapy; cell aggregate; computational fluid dynamics; differentiation; expansion; homogeneous hydrodynamic environment; human embryonic stem cell; induced pluripotent stem cell; medium exchange; oxygenation; scalable manufacturing; scale up; shear stress; single-use bioreactor; turbulent energy dissipation rate; vertical-wheel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

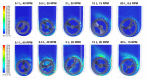
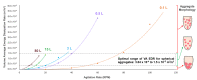

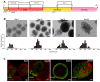
References
-
- Rodrigues C.A.V., Branco M., Nogueira D.E.S., Silva T., Gomes A.R., Diogo M.M., Cabral J.M.S. Bioreactors for Human Pluripotent Stem Cell Expansion and Differentiation. In: Cabral J.M.S., Lobato da Silva C., editors. Bioreactors for Stem Cell Expansion and Differentiation. CRC Press; Boca Raton, FL, USA: 2018. pp. 1–21.
LinkOut - more resources
Full Text Sources

