Distensibility of Deformable Aortic Replicas Assessed by an Integrated In-Vitro and In-Silico Approach
- PMID: 35324783
- PMCID: PMC8945006
- DOI: 10.3390/bioengineering9030094
Distensibility of Deformable Aortic Replicas Assessed by an Integrated In-Vitro and In-Silico Approach
Abstract
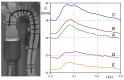
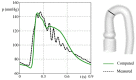
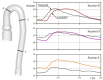
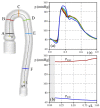
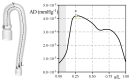
The correct estimation of the distensibility of deformable aorta replicas is a challenging issue, in particular when its local characterization is necessary. We propose a combined in-vitro and in-silico approach to face this problem. First, we tested an aortic silicone arch in a pulse-duplicator analyzing its dynamics under physiological working conditions. The aortic flow rate and pressure were measured by a flow meter at the inlet and two probes placed along the arch, respectively. Video imaging analysis allowed us to estimate the outer diameter of the aorta in some sections in time. Second, we replicated the in-vitro experiment through a Fluid-Structure Interaction simulation. Observed and computed values of pressures and variations in aorta diameters, during the cardiac cycle, were compared. Results were considered satisfactory enough to suggest that the estimation of local distensibility from in-silico tests is reliable, thus overcoming intrinsic experimental limitations. The aortic distensibility (AD) is found to vary significantly along the phantom by ranging from 3.0 × 10-3 mmHg-1 in the ascending and descending tracts to 4.2 × 10-3 mmHg-1 in the middle of the aortic arch. Interestingly, the above values underestimate the AD obtained in preliminary tests carried out on straight cylindrical samples made with the same material of the present phantom. Hence, the current results suggest that AD should be directly evaluated on the replica rather than on the samples of the adopted material. Moreover, tests should be suitably designed to estimate the local rather than only the global distensibility.
Keywords: FSI simulation; aortic compliance; aortic distensibility; aortic phantom; in-vitro experiments; pulse-duplicator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
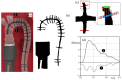


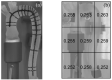
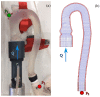
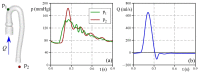
Similar articles
-
Aortic strain, flow pattern and wall shear stress in a patient-specific compliant aorta replica using Shake-the-Box.Med Eng Phys. 2025 Jan;135:104263. doi: 10.1016/j.medengphy.2024.104263. Epub 2024 Nov 26. Med Eng Phys. 2025. PMID: 39922656
-
Aortic hemodynamics assessment prior and after valve sparing reconstruction: A patient-specific 4D flow-based FSI model.Comput Biol Med. 2021 Aug;135:104581. doi: 10.1016/j.compbiomed.2021.104581. Epub 2021 Jun 18. Comput Biol Med. 2021. PMID: 34174756
-
Elastic properties of the young aorta: ex vivo perfusion experiments in a porcine model.Eur J Cardiothorac Surg. 2015 Aug;48(2):221-7. doi: 10.1093/ejcts/ezu438. Epub 2014 Nov 13. Eur J Cardiothorac Surg. 2015. PMID: 25394416
-
[Aortic wall distensibility and the structure and function of the left ventricle in aged persons with isolated systolic hypertension].Srp Arh Celok Lek. 1999 Jan-Feb;127(1-2):10-5. Srp Arh Celok Lek. 1999. PMID: 10377834 Serbian.
-
Cardiovascular characteristics in Marfan syndrome and their relation to the genotype.Verh K Acad Geneeskd Belg. 2009;71(6):335-71. Verh K Acad Geneeskd Belg. 2009. PMID: 20232788 Review.
Cited by
-
Mechanical testing and comparison of porcine tissue, silicones and 3D-printed materials for cardiovascular phantoms.Front Bioeng Biotechnol. 2023 Dec 1;11:1274673. doi: 10.3389/fbioe.2023.1274673. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38107617 Free PMC article.
References
LinkOut - more resources
Full Text Sources

