Extracellular Vesicles in Type 1 Diabetes: A Versatile Tool
- PMID: 35324794
- PMCID: PMC8945706
- DOI: 10.3390/bioengineering9030105
Extracellular Vesicles in Type 1 Diabetes: A Versatile Tool
Abstract
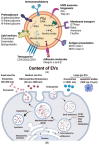
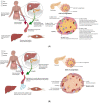
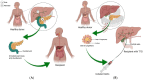
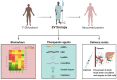
Type 1 diabetes is a chronic autoimmune disease affecting nearly 35 million people. This disease develops as T-cells continually attack the β-cells of the islets of Langerhans in the pancreas, which leads to β-cell death, and steadily decreasing secretion of insulin. Lowered levels of insulin minimize the uptake of glucose into cells, thus putting the body in a hyperglycemic state. Despite significant progress in the understanding of the pathophysiology of this disease, there is a need for novel developments in the diagnostics and management of type 1 diabetes. Extracellular vesicles (EVs) are lipid-bound nanoparticles that contain diverse content from their cell of origin and can be used as a biomarker for both the onset of diabetes and transplantation rejection. Furthermore, vesicles can be loaded with therapeutic cargo and delivered in conjunction with a transplant to increase cell survival and long-term outcomes. Crucially, several studies have linked EVs and their cargos to the progression of type 1 diabetes. As a result, gaining a better understanding of EVs would help researchers better comprehend the utility of EVs in regulating and understanding type 1 diabetes. EVs are a composition of biologically active components such as nucleic acids, proteins, metabolites, and lipids that can be transported to particular cells/tissues through the blood system. Through their varied content, EVs can serve as a flexible aid in the diagnosis and management of type 1 diabetes. In this review, we provide an overview of existing knowledge about EVs. We also cover the role of EVs in the pathogenesis, detection, and treatment of type 1 diabetes and the function of EVs in pancreas and islet β-cell transplantation.
Keywords: EVs; T1DM; biomarkers; exosomes; extracellular vesicles; islet β-cell transplant; microRNA; pancreas transplant; therapy; type 1 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Extracellular Vesicles in Immune System Regulation and Type 1 Diabetes: Cell-to-Cell Communication Mediators, Disease Biomarkers, and Promising Therapeutic Tools.Front Immunol. 2021 Jun 9;12:682948. doi: 10.3389/fimmu.2021.682948. eCollection 2021. Front Immunol. 2021. PMID: 34177928 Free PMC article. Review.
-
Extracellular vesicle-mediated intercellular and interorgan crosstalk of pancreatic islet in health and diabetes.Front Endocrinol (Lausanne). 2023 May 25;14:1170237. doi: 10.3389/fendo.2023.1170237. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37305058 Free PMC article. Review.
-
Circulating Extracellular Vesicles As Biomarkers and Drug Delivery Vehicles in Cardiovascular Diseases.Biomolecules. 2021 Mar 5;11(3):388. doi: 10.3390/biom11030388. Biomolecules. 2021. PMID: 33808038 Free PMC article. Review.
-
Extracellular vesicles from human pancreatic islets suppress human islet amyloid polypeptide amyloid formation.Proc Natl Acad Sci U S A. 2017 Oct 17;114(42):11127-11132. doi: 10.1073/pnas.1711389114. Epub 2017 Oct 2. Proc Natl Acad Sci U S A. 2017. PMID: 28973954 Free PMC article.
-
The Role of Extracellular Vesicles in β-Cell Function and Viability: A Scoping Review.Front Endocrinol (Lausanne). 2020 Jun 11;11:375. doi: 10.3389/fendo.2020.00375. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32595604 Free PMC article.
Cited by
-
A negative regulatory role of β-cell-derived exosomes in the glucose-stimulated insulin secretion of recipient β-cells.Arch Toxicol. 2024 Nov;98(11):3885-3896. doi: 10.1007/s00204-024-03838-8. Epub 2024 Aug 10. Arch Toxicol. 2024. PMID: 39127846
-
Roles of extracellular vesicles associated non-coding RNAs in Diabetes Mellitus.Front Endocrinol (Lausanne). 2022 Dec 22;13:1057407. doi: 10.3389/fendo.2022.1057407. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36619588 Free PMC article. Review.
-
Fighting Fire with Fire: Exosomes and Acute Pancreatitis-Associated Acute Lung Injury.Bioengineering (Basel). 2022 Oct 26;9(11):615. doi: 10.3390/bioengineering9110615. Bioengineering (Basel). 2022. PMID: 36354526 Free PMC article. Review.
-
The impact of environmental contaminants on extracellular vesicles and their key molecular regulators: A literature and database-driven review.Environ Mol Mutagen. 2023 Jan;64(1):50-66. doi: 10.1002/em.22522. Epub 2022 Dec 25. Environ Mol Mutagen. 2023. PMID: 36502378 Free PMC article. Review.
-
Extracellular Vesicles in Sickle Cell Disease: A Promising Tool.Bioengineering (Basel). 2022 Sep 5;9(9):439. doi: 10.3390/bioengineering9090439. Bioengineering (Basel). 2022. PMID: 36134985 Free PMC article. Review.
References
-
- Wolkowicz K.L., Aiello E.M., Vargas E., Teymourian H., Tehrani F., Wang J., Pinsker J.E., Doyle F.J., III, Patti M.E., Laffel L.M., et al. A review of biomarkers in the context of type 1 diabetes: Biological sensing for enhanced glucose control. Bioeng. Transl. Med. 2021;6:e10201. doi: 10.1002/btm2.10201. - DOI - PMC - PubMed
-
- Subramanian S.A.B.D. In: The Management of Type 1 Diabetes, in Comprehensive Free Online Endocrinology Book. Feingold K.R., Anawalt B., Boyce A., editors. MDText.com; South Dartmouth, MA, USA: 2000.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

