Present Application and Perspectives of Organoid Imaging Technology
- PMID: 35324810
- PMCID: PMC8945799
- DOI: 10.3390/bioengineering9030121
Present Application and Perspectives of Organoid Imaging Technology
Abstract
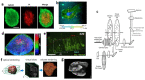
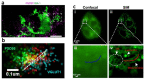
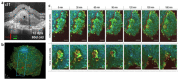
An organoid is a miniaturized and simplified in vitro model with a similar structure and function to a real organ. In recent years, the use of organoids has increased explosively in the field of growth and development, disease simulation, drug screening, cell therapy, etc. In order to obtain necessary information, such as morphological structure, cell function and dynamic signals, it is necessary and important to directly monitor the culture process of organoids. Among different detection technologies, imaging technology is a simple and convenient choice and can realize direct observation and quantitative research. In this review, the principle, advantages and disadvantages of imaging technologies that have been applied in organoids research are introduced. We also offer an overview of prospective technologies for organoid imaging. This review aims to help biologists find appropriate imaging techniques for different areas of organoid research, and also contribute to the development of organoid imaging systems.
Keywords: imaging technology; microscopy; optical coherence tomography; organoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
[A review on depth perception techniques in organoid images].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2024 Oct 25;41(5):1053-1061. doi: 10.7507/1001-5515.202404036. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2024. PMID: 39462675 Free PMC article. Review. Chinese.
-
Automated detection and growth tracking of 3D bio-printed organoid clusters using optical coherence tomography with deep convolutional neural networks.Front Bioeng Biotechnol. 2023 Apr 12;11:1133090. doi: 10.3389/fbioe.2023.1133090. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37122853 Free PMC article.
-
Progress and perspective of organoid technology in cancer-related translational medicine.Biomed Pharmacother. 2022 May;149:112869. doi: 10.1016/j.biopha.2022.112869. Epub 2022 Mar 28. Biomed Pharmacother. 2022. PMID: 35358798 Review.
-
A review of protocols for brain organoids and applications for disease modeling.STAR Protoc. 2023 Mar 17;4(1):101860. doi: 10.1016/j.xpro.2022.101860. Epub 2022 Dec 24. STAR Protoc. 2023. PMID: 36566384 Free PMC article. Review.
-
Advances in Central Nervous System Organoids: A Focus on Organoid-Based Models for Motor Neuron Disease.Tissue Eng Part C Methods. 2021 Mar;27(3):213-224. doi: 10.1089/ten.TEC.2020.0337. Epub 2021 Mar 3. Tissue Eng Part C Methods. 2021. PMID: 33446055 Review.
Cited by
-
Engineered organoids for biomedical applications.Adv Drug Deliv Rev. 2023 Dec;203:115142. doi: 10.1016/j.addr.2023.115142. Epub 2023 Nov 13. Adv Drug Deliv Rev. 2023. PMID: 37967768 Free PMC article. Review.
-
Long-term three-dimensional high-resolution imaging of live unlabeled small intestinal organoids via low-coherence holotomography.Exp Mol Med. 2024 Oct;56(10):2162-2170. doi: 10.1038/s12276-024-01312-0. Epub 2024 Oct 1. Exp Mol Med. 2024. PMID: 39349827 Free PMC article.
-
Gastrointestinal tract, its pathophysiology and in-vitro models: A "quick" reference guide to translational studies.World J Gastroenterol. 2025 Jul 28;31(28):108297. doi: 10.3748/wjg.v31.i28.108297. World J Gastroenterol. 2025. PMID: 40741472 Free PMC article. Review.
-
Experimental and numerical investigation of wavelength and resolution dependency of dynamic optical coherence tomography signals.Biomed Opt Express. 2025 Jul 7;16(8):3084-3104. doi: 10.1364/BOE.564030. eCollection 2025 Aug 1. Biomed Opt Express. 2025. PMID: 40809984 Free PMC article.
-
How to Study the Mechanobiology of Intestinal Epithelial Organoids? A Review of Culture Supports, Imaging Techniques, and Analysis Methods.Biol Cell. 2025 Apr;117(4):e70003. doi: 10.1111/boc.70003. Biol Cell. 2025. PMID: 40223609 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

