Vascularized Co-Culture Clusteroids of Primary Endothelial and Hep-G2 Cells Based on Aqueous Two-Phase Pickering Emulsions
- PMID: 35324815
- PMCID: PMC8945860
- DOI: 10.3390/bioengineering9030126
Vascularized Co-Culture Clusteroids of Primary Endothelial and Hep-G2 Cells Based on Aqueous Two-Phase Pickering Emulsions
Abstract
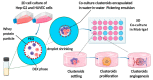
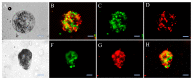
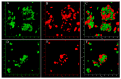
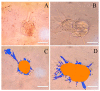
Three-dimensional cell culture has been extensively involved in biomedical applications due to its high availability and relatively mature biochemical properties. However, single 3D cell culture models based on hydrogel or various scaffolds do not meet the more in-depth requirements of in vitro models. The necrotic core formation inhibits the utilization of the 3D cell culture ex vivo as oxygen permeation is impaired in the absence of blood vessels. We report a simple method to facilitate the formation of angiogenic HUVEC (human umbilical vein endothelial cells) and Hep-G2 (hepatocyte carcinoma model) co-culture 3D clusteroids in a water-in-water (w/w) Pickering emulsions template which can overcome this limitation. This method enabled us to manipulate the cells proportion in order to achieve the optimal condition for stimulating the production of various angiogenic protein markers in the co-cultured clusteroids. The HUVEC cells respond to the presence of Hep-G2 cells and their byproducts by forming endothelial cell sprouts in Matrigel without the exogenous addition of vascular endothelial growth factor (VEGF) or other angiogenesis inducers. This culture method can be easily replicated to produce other types of cell co-culture spheroids. The w/w Pickering emulsion template can facilitate the fabrication of 3D co-culture models to a great extent and be further utilized in drug testing and tissue engineering applications.
Keywords: 3D cell culture; angiogenesis; co-culture; water-in-water Pickering emulsions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Fabrication of Angiogenic Sprouting Coculture of Cell Clusteroids Using an Aqueous Two-Phase Pickering Emulsion System.ACS Appl Bio Mater. 2022 Apr 18;5(4):1804-1816. doi: 10.1021/acsabm.2c00168. Epub 2022 Mar 22. ACS Appl Bio Mater. 2022. PMID: 35315278
-
Fabrication of Human Keratinocyte Cell Clusters for Skin Graft Applications by Templating Water-in-Water Pickering Emulsions.Biomimetics (Basel). 2019 Jul 11;4(3):50. doi: 10.3390/biomimetics4030050. Biomimetics (Basel). 2019. PMID: 31336810 Free PMC article.
-
Biodegradable Pickering emulsions of Lipiodol for liver trans-arterial chemo-embolization.Acta Biomater. 2019 Mar 15;87:177-186. doi: 10.1016/j.actbio.2019.01.054. Epub 2019 Jan 30. Acta Biomater. 2019. PMID: 30708065
-
Bioengineered three-dimensional co-culture of cancer cells and endothelial cells: A model system for dual analysis of tumor growth and angiogenesis.Biotechnol Bioeng. 2017 Aug;114(8):1865-1877. doi: 10.1002/bit.26297. Epub 2017 May 8. Biotechnol Bioeng. 2017. PMID: 28369747
-
DPSCs treated by TGF-β1 regulate angiogenic sprouting of three-dimensionally co-cultured HUVECs and DPSCs through VEGF-Ang-Tie2 signaling.Stem Cell Res Ther. 2021 May 10;12(1):281. doi: 10.1186/s13287-021-02349-y. Stem Cell Res Ther. 2021. PMID: 33971955 Free PMC article.
Cited by
-
Decellularized liver scaffolds for constructing drug-metabolically functional ex vivo human liver models.Bioact Mater. 2024 Sep 23;43:162-180. doi: 10.1016/j.bioactmat.2024.09.029. eCollection 2025 Jan. Bioact Mater. 2024. PMID: 39386220 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

