Molecular Mechanisms of Lipopolysaccharide (LPS) Induced Inflammation in an Immortalized Ovine Luteal Endothelial Cell Line (OLENDO)
- PMID: 35324827
- PMCID: PMC8950530
- DOI: 10.3390/vetsci9030099
Molecular Mechanisms of Lipopolysaccharide (LPS) Induced Inflammation in an Immortalized Ovine Luteal Endothelial Cell Line (OLENDO)
Abstract
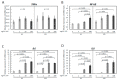
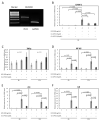
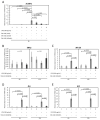
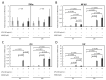
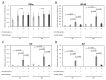
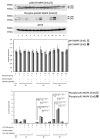
Escherichia coli (E. coli) is the most common Gram-negative bacterium causing infection of the uterus or mammary gland and is one of the major causes of infertility in livestock. In those animals affected by E. coli driven LPS-mediated infections, fertility problems occur in part due to disrupted follicular and luteal functionality. However, the molecular mechanisms by which LPS induces inflammation, and specifically, the role of LPS in the disruption of capillary morphogenesis and endothelial barrier function remain unclear. Here, we hypothesized that LPS may lead to alterations in luteal angiogenesis and vascular function by inducing inflammatory reactions in endothelial cells. Accordingly, OLENDO cells were treated with LPS followed by evaluation of the expression of selected representative proinflammatory cytokines: NF-kB, IL6, IL8, TNFα, and ICAM 1. While TNFα was not affected by treatment with LPS, transcripts of NF-kB, IL6, and IL8 were affected in a dosage-dependent manner. Additionally, the activity of TLR2 and TLR4 was blocked, resulting in suppression of the LPS-induced expression of ICAM 1, NF-kB, IL6, and IL8. Inhibition of the PKA or MAPK/ERK pathways suppressed the LPS-stimulated expression of NF-kB, IL6, and IL8, whereas blocking the PKC pathway had the opposite effect. Furthermore, LPS-induced phosphorylation of Erk1 and Erk2 was inhibited when the TLR4 or MAPK/ERK pathways were blocked. Finally, LPS seems to induce inflammatory processes in OLENDO cells via TLR2 and TLR4, utilizing different signaling pathways.
Keywords: MAPKs; PKA; PKC; inflammation; interleukins; ovary; toll-like receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sheldon I.M., Rycroft A.N., Dogan B., Craven M., Bromfield J.J., Chandler A., Roberts M.H., Price S.B., Gilbert R.O., Simpson K.W. Specific strains of Escherichia coli are pathogenic for the endometrium of cattle and cause pelvic inflammatory disease in cattle and mice. PLoS ONE. 2010;5:e9192. doi: 10.1371/journal.pone.0009192. - DOI - PMC - PubMed
-
- Hertl J.A., Grohn Y.T., Leach J.D., Bar D., Bennett G.J., Gonzalez R.N., Rauch B.J., Welcome F.L., Tauer L.W., Schukken Y.H. Effects of clinical mastitis caused by gram-positive and gram-negative bacteria and other organisms on the probability of conception in New York State Holstein dairy cows. J. Dairy Sci. 2010;93:1551–1560. doi: 10.3168/jds.2009-2599. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

