Use of Electrodiagnostics in the Diagnosis and Follow-Up of Brachial Plexus Syndrome in a Calf
- PMID: 35324865
- PMCID: PMC8950725
- DOI: 10.3390/vetsci9030136
Use of Electrodiagnostics in the Diagnosis and Follow-Up of Brachial Plexus Syndrome in a Calf
Abstract
Electrodiagnostic testing by using electromyography (EMG) and nerve conduction studies (NCS) is essential in the evaluation of patients with traumatic brachial plexus injury as it facilitates the localization of the lesion and the prognosis. In this case report, we present a long-term electrodiagnostic follow-up of a 5-day-old female Holstein calf with brachial plexus syndrome. Electrodiagnostic studies were carried out at 2 weeks, 5 weeks, 7 months and 12 months after admission. Initially, EMG confirmed the damage to the brachial plexus, potentially indicating a condition of neurotmesis or axonotmesis. However, motor NCS and the repeated electrodiagnostic follow-up, along with the evolution of the clinical signs, allowed a more favorable diagnosis of axonotmesis to be made. In fact, EMG showed a slow but gradual reduction and finally the disappearance of spontaneous pathological activity, while motor NCS revealed an increase in the amplitude and areas of the compound muscle action potentials. The animal was deemed fully recovered 12 months after admission. To the authors' knowledge, this is the first report on the use of motor NCS in bovine medicine and it demonstrates that electrodiagnostics represent a useful and practical tool for the evaluation and prognosis of brachial plexus injury cases in cattle.
Keywords: bovine; brachial plexus; electromyography; motor nerve conduction study; neurology; traumatic injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

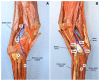

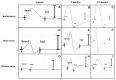
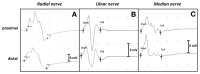
References
-
- Lorenz M.D., Coates J.R., Kent M. Handbook of Veterinary Neurology. 5th ed. Elsevier-Saunders; St. Louis, MO, USA: 2011. pp. 94–108.
-
- Barone R., Simoens P. Anatomia Comparata dei Mammiferi Domestici—Vol. 7° Neurologia. 1st ed. Edagricole New Business Media; Milano, Italy: 2012. pp. 133–275.
-
- Greenough P.R., Weaver A.D. Lameness in Cattle. 3rd ed. Saunders; Philadelphia, PA, USA: 1997. pp. 203–218.
-
- De Lahunta A., Glass E. Veterinary Neuroanatomy and Clinical Neurology. 3rd ed. Saunders-Elsevier; St. Louis, MO, USA: 2009. pp. 77–133.
Publication types
LinkOut - more resources
Full Text Sources

