Preliminary Bioequivalence of an Oral Pimobendan Solution Formulation with Reference Solution Formulation in Beagle Dogs
- PMID: 35324869
- PMCID: PMC8955067
- DOI: 10.3390/vetsci9030141
Preliminary Bioequivalence of an Oral Pimobendan Solution Formulation with Reference Solution Formulation in Beagle Dogs
Abstract
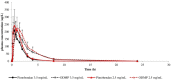
Oral capsule and tablet formulations of pimobendan are widely used but may present difficulties for accurately dosing small patients. This study aimed to compare the pharmacokinetic (PK) characteristics, bioequivalence, and cardiovascular effects of a custom-made oral pimobendan solution (PS) formulation compared to a reference solution (RS) formulation in conscious, healthy dogs. A randomized crossover design was performed on dogs that received RS and PS formulations at a dose of 0.3 mg/kg. Blood samples were collected at 0, 0.083, 0.167, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 8, and 24 h after oral administration for PK analysis; bioequivalence was also calculated. Echocardiography was also performed to assess the cardiovascular effects. The results revealed that the plasma concentrations of pimobendan and o-desmethyl-pimobendan (active metabolite) in the case of both formulations were comparable. The relative ratios of geometric mean concentrations for all significant parameters of PK were within a range of 80-125%, indicating bioequivalence. In addition, both formulations increased cardiac contraction significantly when compared with the baseline, and no differences in cardiac contractility were detected between the formulations. The PS formulation can be used as alternative to the RS formulation for the management of congestive heart disease because of the bioequivalence between the two formulations.
Keywords: ODMP; bioequivalence; cardiac function; dog; echocardiography; oral; pharmacodynamics; pharmacokinetics; pimobendan; solution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Pimobendan oral solution is bioequivalent to pimobendan chewable tablets in beagle dogs.J Vet Intern Med. 2025 Jan-Feb;39(1):e17248. doi: 10.1111/jvim.17248. J Vet Intern Med. 2025. PMID: 39835518 Free PMC article.
-
Pharmacodynamics and Pharmacokinetics of Injectable Pimobendan and Its Metabolite, O-Desmethyl-Pimobendan, in Healthy Dogs.Front Vet Sci. 2021 Aug 20;8:656902. doi: 10.3389/fvets.2021.656902. eCollection 2021. Front Vet Sci. 2021. PMID: 34490386 Free PMC article.
-
Pharmacokinetics of Pimobendan and Its Metabolite O-Desmethyl-Pimobendan Following Rectal Administration to Healthy Dogs.Front Vet Sci. 2020 Aug 4;7:423. doi: 10.3389/fvets.2020.00423. eCollection 2020. Front Vet Sci. 2020. PMID: 32851013 Free PMC article.
-
Single-dose pharmacokinetics and cardiovascular effects of oral pimobendan in healthy cats.J Vet Cardiol. 2016 Dec;18(4):310-325. doi: 10.1016/j.jvc.2016.07.001. Epub 2016 Sep 6. J Vet Cardiol. 2016. PMID: 27613648 Clinical Trial.
-
The pharmacokinetics of pimobendan enantiomers after oral and intravenous administration of racemate pimobendan formulations in healthy dogs.J Vet Pharmacol Ther. 2016 Feb;39(1):54-61. doi: 10.1111/jvp.12235. Epub 2015 May 18. J Vet Pharmacol Ther. 2016. PMID: 25989021
References
-
- Boswood A., Gordon S.G., Haggstrom J., Wess G., Stepien R.L., Oyama M.A., Keene B.W., Bonagura J., MacDonald K.A., Patteson M., et al. Longitudinal Analysis of Quality of Life, Clinical, Radiographic, Echocardiographic, and Laboratory Variables in Dogs with Preclinical Myxomatous Mitral Valve Disease Receiving Pimobendan or Placebo: The EPIC Study. J. Vet. Intern. Med. 2018;32:72–85. doi: 10.1111/jvim.14885. - DOI - PMC - PubMed
-
- Boswood A., Haggstrom J., Gordon S.G., Wess G., Stepien R.L., Oyama M.A., Keene B.W., Bonagura J., MacDonald K.A., Patteson M., et al. Effect of Pimobendan in Dogs with Preclinical Myxomatous Mitral Valve Disease and Cardiomegaly: The EPIC Study-A Randomized Clinical Trial. J. Vet. Intern. Med. 2016;30:1765–1779. doi: 10.1111/jvim.14586. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources