Human Muse cells reduce myocardial infarct size and improve cardiac function without causing arrythmias in a swine model of acute myocardial infarction
- PMID: 35324926
- PMCID: PMC8947423
- DOI: 10.1371/journal.pone.0265347
Human Muse cells reduce myocardial infarct size and improve cardiac function without causing arrythmias in a swine model of acute myocardial infarction
Abstract
Background: We recently reported that multilineage-differentiating stress enduring (Muse) cells intravenously administered after acute myocardial infarction (AMI), selectively engrafted to the infarct area, spontaneously differentiated into cardiomyocytes and vessels, reduced the infarct size, improved the left ventricular (LV) function and remodeling in rabbits. We aimed to clarify the efficiency of Muse cells in a larger animal AMI model of mini-pigs using a semi-clinical grade human Muse cell product.
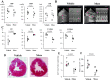
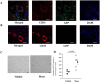
Method and result: Mini-pigs underwent 30 min of coronary artery occlusion followed by 2 weeks of reperfusion. Semi-clinical grade human Muse cell product (1x107, Muse group, n = 5) or saline (Vehicle group, n = 7) were intravenously administered at 24 h after reperfusion. The infarct size, LV function and remodeling were evaluated by echocardiography. Arrhythmias were evaluated by an implantable loop recorder. The infarct size was significantly smaller in the Muse group (10.5±3.3%) than in the Vehicle group (21.0±2.0%). Both the LV ejection fraction and fractional shortening were significantly greater in the Muse group than in the Vehicle group. The LV end-systolic and end-diastolic dimensions were significantly smaller in the Muse group than in the Vehicle group. Human Muse cells homed into the infarct border area and expressed cardiac troponin I and vascular endothelial CD31. No arrhythmias and no blood test abnormality were observed.
Conclusion: Muse cell product might be promising for AMI therapy based on the efficiency and safety in a mini-pig AMI.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Sphingosine-1-Phosphate Receptor 2 Agonist Mobilises Endogenous Muse Cells to Repair Damaged Myocardial Tissue in Male Rabbits.J Cell Mol Med. 2025 Apr;29(8):e70447. doi: 10.1111/jcmm.70447. J Cell Mol Med. 2025. PMID: 40245180 Free PMC article.
-
Stem cell therapy for acute myocardial infarction - focusing on the comparison between Muse cells and mesenchymal stem cells.J Cardiol. 2022 Jul;80(1):80-87. doi: 10.1016/j.jjcc.2021.10.030. Epub 2021 Dec 17. J Cardiol. 2022. PMID: 34924234 Review.
-
Acute Myocardial Infarction, Cardioprotection, and Muse Cells.Adv Exp Med Biol. 2018;1103:153-166. doi: 10.1007/978-4-431-56847-6_8. Adv Exp Med Biol. 2018. PMID: 30484228 Review.
-
Mobilized Muse Cells After Acute Myocardial Infarction Predict Cardiac Function and Remodeling in the Chronic Phase.Circ J. 2018 Jan 25;82(2):561-571. doi: 10.1253/circj.CJ-17-0552. Epub 2017 Sep 20. Circ J. 2018. PMID: 28931784 Clinical Trial.
-
S1P-S1PR2 Axis Mediates Homing of Muse Cells Into Damaged Heart for Long-Lasting Tissue Repair and Functional Recovery After Acute Myocardial Infarction.Circ Res. 2018 Apr 13;122(8):1069-1083. doi: 10.1161/CIRCRESAHA.117.311648. Epub 2018 Feb 23. Circ Res. 2018. PMID: 29475983
Cited by
-
Safety and Clinical Effects of a Muse Cell-Based Product in Patients With Amyotrophic Lateral Sclerosis: Results of a Phase 2 Clinical Trial.Cell Transplant. 2023 Jan-Dec;32:9636897231214370. doi: 10.1177/09636897231214370. Cell Transplant. 2023. PMID: 38014622 Free PMC article. Clinical Trial.
-
Multilineage-Differentiating Stress-Enduring Cells (Muse Cells): An Easily Accessible, Pluripotent Stem Cell Niche with Unique and Powerful Properties for Multiple Regenerative Medicine Applications.Biomedicines. 2023 May 30;11(6):1587. doi: 10.3390/biomedicines11061587. Biomedicines. 2023. PMID: 37371682 Free PMC article. Review.
-
Detection, Isolation and Quantification of Myocardial Infarct with Four Different Histological Staining Techniques.Diagnostics (Basel). 2024 Oct 18;14(20):2325. doi: 10.3390/diagnostics14202325. Diagnostics (Basel). 2024. PMID: 39451648 Free PMC article.
-
Multilineage-differentiating stress-enduring cells: a powerful tool for tissue damage repair.Front Cell Dev Biol. 2024 May 30;12:1380785. doi: 10.3389/fcell.2024.1380785. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38872932 Free PMC article. Review.
-
Macrophage- and pluripotent-like reparative Muse cells are unique endogenous stem cells distinct from other somatic stem cells.Front Bioeng Biotechnol. 2025 Mar 27;13:1553382. doi: 10.3389/fbioe.2025.1553382. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40213632 Free PMC article. Review.
References
-
- Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M et al.. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H2670–H2676 doi: 10.1152/ajpheart.01071.2003 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

